Small Molecules Discovered to Halt Cellular Aging
Scientists from Boston Children's Hospital identify potential drugs for treating telomere diseases, and maybe even aging.
Sitting at the end of strands of DNA, telomeres play a crucial role in cellular aging. Like the protective caps on shoelaces, telomeres safeguard our DNA from damages during cell division. Unfortunately, these caps shorten and fray as the cell ages, and our cells inevitably become frail and die.
But researchers from Harvard University found small molecules that can restore the activity of telomerase, a telomere-lengthening enzyme, to rescue telomere length. The study published in Cell Stem Cell may pave the way to treatments for illnesses ranging from rare telomere diseases to aging.
Sifting through over 100,000 small molecules, the researchers identified one novel compound — BCH001 — that appeared to significantly reverse the cellular aging process in cells with dyskeratosis congenita. Dyskeratosis congenita is a rare genetic disease known as short telomere disease. It disrupts the formation and function of telomerase, an enzyme critical for the integrity of telomeres, resulting in prematurely aged cells.
“Once human telomerase was identified, there were lots of biotech startups, lots of investment,” said correspondent author Suneet Agarwal of Dana-Farber/Boston Children’s Cancer and Blood Disorders Center in a press release. “But it didn’t pan out. There are no drugs on the market, and companies have come and gone.”
For patients with dyskeratosis congenita, there are no curative therapies besides bone marrow or organ transplantation. However, the treatment is high-risk, and the outcome is poor due to the patients’ impaired regenerative capacity and tissue function. Agarwal and the team saw a need for a systemic therapy to safely restore telomere maintenance and moved towards the field of small molecule therapeutics for telomere diseases.
Scientists have identified two molecules — PAPD5 and PARN — that control the maturation and degradation of a key telomerase component called TERC. Acting as opposing forces of yin and yang, PAPD5 leads the immature telomerase down the path of degradation, while PARN leads it down the maturation pathway to become telomerase. But one of the gene mutations in telomere diseases depletes the level of PARN. With the drop of the level in PARN, the molecular scale tilts towards PAPD5, leading telomerase towards degradation and leaving the cell with nothing to repair the damaged telomeres.
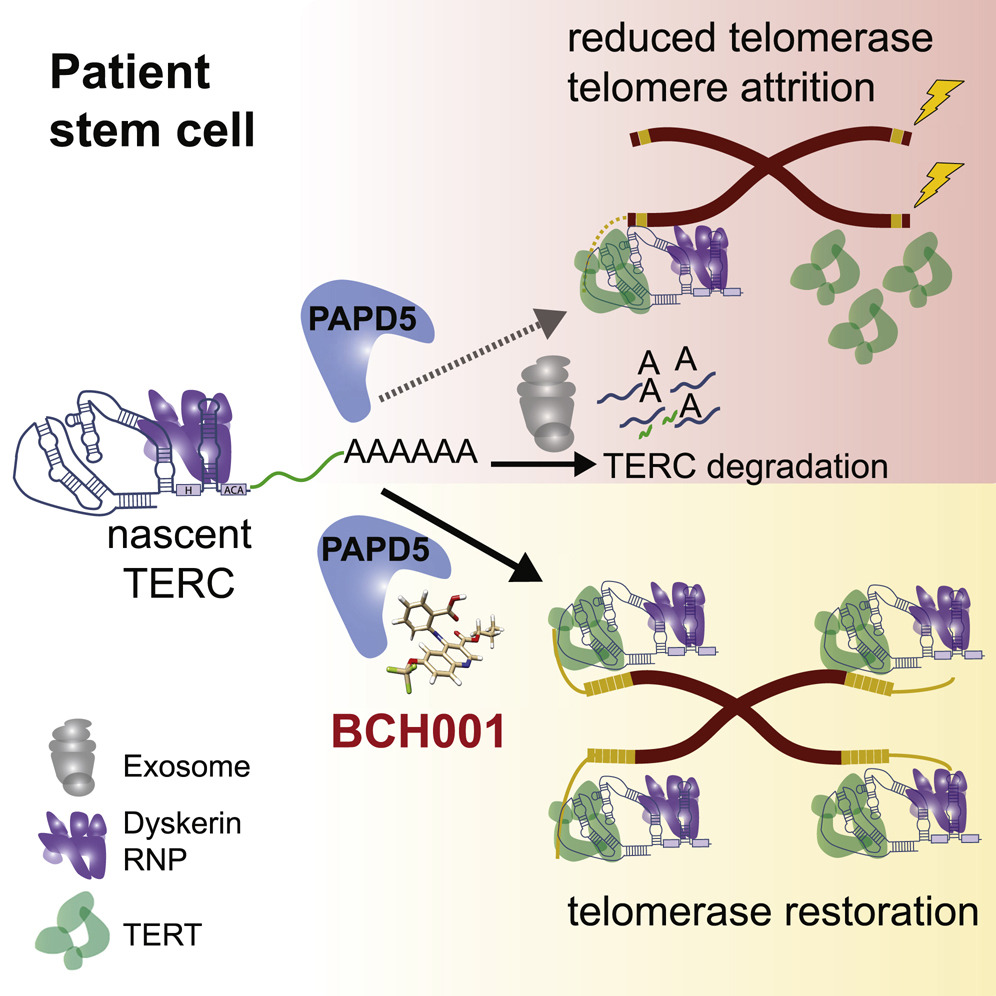
“We thought if we targeted PAPD5, we could protect TERC and restore the proper balance of telomerase,” said the author Neha Nagpal of Dana-Farber/Boston Children’s Hospital in a press release.
The PAPD5 inhibitor, BCH001, restored telomerase activity, and telomere length in dyskeratosis congenita stem cells. However, the restoring effect disappeared when the researchers removed the small molecule, suggesting ongoing telomere maintenance requires PAPD5 inhibition.
To test if the treatment would be safe, the team transplanted human blood stem cells with telomere-disease-causing mutations into mice and treated them with oral PAPD5 inhibitor, RG7834. The orally distributed molecule not only boosted the level of matured TERC but also restored telomere length in the transplanted stem cells without adverse effects.
“This provided the hope that this could become a clinical treatment,” said Nagpal in the press release. The study identified BCH001 and RG7834 as promising leads of a new class of telomere therapeutics.
“We expect restoring telomeres in stem cells will increase tissue regenerative capacity in the blood, lungs, and other organs affected in [dyskeratosis congenita] and other diseases,” Argawal added in the press release. The next step for the team is to determine the potential of PAPD5 inhibition in other diseases caused by defects in telomere maintenance, and perhaps even aging itself.