Stanford Scientists Identify Molecule That Rejuvenates Stem Cells and Restores Strength in Aging Muscles
New study finds that a single dose of a natural hormone analog restores strength and stem cell function in aged muscle, offering hope for reversing age-related decline.
Highlights:
- A single dose of prostaglandin E₂ rejuvenated aged muscle stem cells in mice, significantly improving muscle strength, mass, and regeneration.
- Blocking prostaglandin E₂ signaling in muscle stem cells accelerated age-related muscle decline, demonstrating its essential role in preserving muscle health during aging.
As we get older, even the simple act of standing up from a chair or walking up the stairs can start to feel different. The muscle power we once took for granted begins to fade, slowly and unmistakably. Behind this decline is a quiet but critical shift happening deep within our tissue: the gradual breakdown of muscle stem cell function.
Muscle stem cells(MuSCs) are the body’s built-in repair crew. They remain dormant until activated by injury, exertion, or disease, at which point they regenerate damaged fibers and support muscle recovery. With age, these cells lose their ability to divide, rebuild, and survive. Over time, their decline contributes to the shrinking strength and slower healing we associate with aging.
A molecule called prostaglandin E₂ (PGE₂) may offer a way to restore MuSCs. Known for its role in inflammation and tissue repair, PGE₂ is released in response to muscle damage and helps activate the regenerative process. In new research from Stanford University, a short burst of PGE₂ in aged mice was enough to awaken dormant stem cells, rebuild muscle fibers, and significantly improve strength.
This rejuvenating effect lasted well beyond the treatment window, suggesting that PGE₂ can create lasting biological change, offering a potential new tool in the fight against age-related muscle decline.
PGE₂ Deficiency in Aging Triggers Muscle Loss
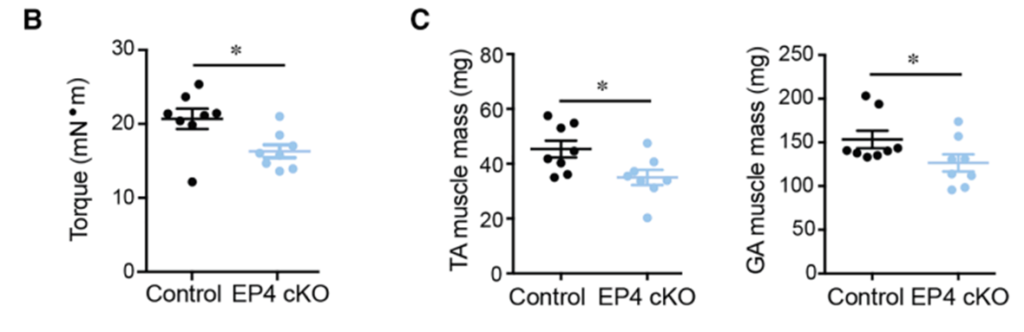
The researchers began by asking whether the natural decline in PGE₂ signaling contributes to muscle deterioration during aging. Using a genetic mouse model that deleted PGE₂’s EP4 receptor in muscle stem cells, they found that mice lacking this pathway lost about 20% of their strength and showed a significant reduction in leg muscle mass as they aged.
This premature muscle decline closely resembled what’s typically seen in older animals, suggesting that PGE₂ is essential for preserving both muscle size and strength across the lifespan.
A Short-Term Boost, Long-Term Results
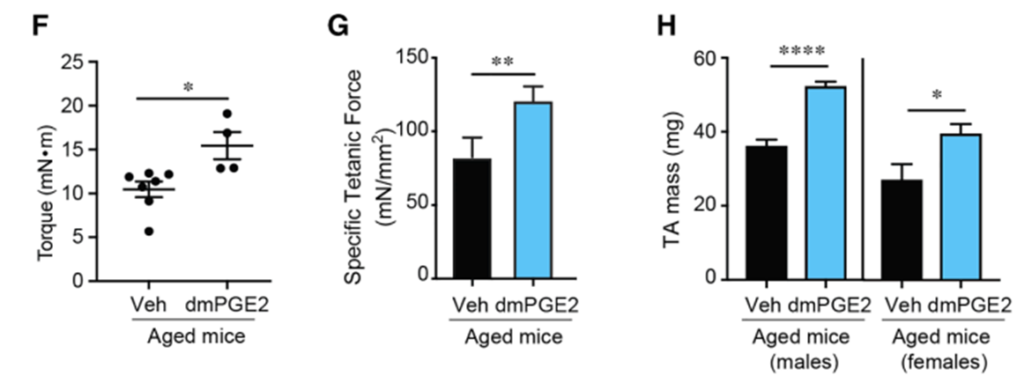
Next, the team tested whether replenishing PGE₂ could reverse age-related muscle decline. Aged mice were treated with a short five-day course of a stable PGE₂ analog, followed by two weeks of mild downhill running. This exercise is known to activate muscle remodeling through eccentric stress, where a muscle lengthens during a contraction.
At the end of the protocol, mice treated with PGE₂ showed dramatic improvements in muscle strength compared to untreated controls. Despite the brief treatment window, the leg muscles of the aged mice were stronger and more resilient, hinting at a lasting effect triggered by PGE₂.
Enhanced Regeneration from Within
To examine how PGE₂ affects muscle regeneration after injury, the researchers injected aged mice with a single dose of PGE₂ two days after muscle damage. Two weeks later, the treated mice had more activated muscle stem cells, larger regenerated fibers, and increased muscle mass and strength.
Remarkably, just one injection of PGE₂ was enough to trigger widespread regeneration in aged tissue. This suggests that even a brief intervention can set off a powerful cascade of stem cell expansion and tissue repair without depleting the stem cell pool.
Rewiring Stem Cells at the Source
In another set of experiments, aged MuSCs were treated with PGE₂ outside the body and then transplanted into the injured muscles of young recipient mice. These pre-treated aged cells showed better long-term engraftment, persisted for weeks, and responded robustly to a second round of injury.
In fact, the transplanted aged cells acted more like young ones, expanding, rebuilding muscle fibers, and even replenishing the stem cell pool. This regenerative boost was lost if the EP4 receptor was removed, confirming that PGE₂ needs this signaling pathway to work.
Implications for Aging and Repair
Although these experiments were conducted in mice, the findings carry significant implications for aging humans. More than 15% of adults over 60 experience sarcopenia, a decline in strength that impairs mobility and quality of life. A similarly brief PGE₂ intervention could one day enhance muscle repair after falls, illness, or exercise, reducing injury risk and strengthening resilience in aging individuals. It also offers hope for those with muscle-wasting diseases like Duchenne muscular dystrophy.
What’s Next?
The Stanford team is already investigating whether PGE₂ delivers similar rejuvenation effects in human muscle stem cells from older individuals, including those with sarcopenia. Their goal is to identify those who may benefit most and to adapt PGE₂ treatment to human clinical contexts.
They also note that while “toxins” can induce muscle injury in mice, exercise-based injury—more representative of everyday strain in humans—elicits the same rejuvenation when paired with PGE₂. Future clinical trials may explore whether older adults can regain the regenerative edge that younger people maintain with routine physical activity.
A Step Forward in Longevity Science
This study contributes to a growing understanding of how short-term molecular therapies can reverse hallmarks of aging, specifically, the loss of muscle stem cell function. By restoring their function and survival, PGE₂ points to a promising new path for repairing muscle and maintaining strength in old age.
Model: Aged mice (Tibialis anterior muscle injury model)
Dosage: 1 µg dmPGE₂ per muscle, injected once 2 days post-injury

