Stem Cell–Derived Vesicles Rejuvenate Pancreas Cells and Cure Age-Related Diabetes
Stem cell–derived vesicles rejuvenate aging pancreatic β cells, restore insulin secretion, and reverse diabetes in old mice by reducing cellular senescence and improving mitochondrial function.
Highlights
- Tiny biological particles released by stem cells restore function in aging insulin-producing cells and reverse diabetes in old mice.
- The treatment reduces cellular senescence, a state in which cells stop working properly and promote inflammation.
- The vesicles improve mitochondrial health, allowing cells to regain normal insulin secretion.
- The benefits are driven by a small regulatory RNA that dampens chronic inflammatory signaling linked to aging.
Aging is one of the strongest risk factors for type 2 diabetes. As we grow older, insulin-producing β cells in the pancreas gradually lose their identity and function. Many of these cells enter cellular senescence, a stressed state in which cells stop dividing, secrete inflammatory signals, and disrupt surrounding tissue. Clearing senescent cells can improve metabolism, but doing so often reduces the total number of β cells, creating new problems.
In a study published in Aging Cell, Xiao and colleagues from Nanjing Medical University describe a different strategy. Instead of removing senescent cells, the researchers use small extracellular vesicles – nano-sized particles naturally released by stem cells – to restore β-cell health and reverse age-related diabetes in mice
These vesicles were isolated from human amniotic membrane mesenchymal stem cells, an ethically accessible stem cell source obtained from discarded placental tissue after birth. Importantly, the treatment is cell-free, meaning no live stem cells were transplanted.
Vesicles Reverse Aging in Insulin-Producing Cells
To test whether the vesicles could counteract aging, the researchers first exposed young mouse β cells to oxidative stress, a driver of cellular aging. These stressed cells, which model aging, displayed signs of senescence, including DNA damage, growth arrest, and loss of insulin production.
When treated with the stem cell–derived vesicles, the β cells showed a marked reversal of these changes. Senescence markers declined, cell growth resumed, and insulin production recovered. Similar improvements were seen in pancreas cells taken from naturally aged mice, demonstrating that the effect was not limited to artificial stress conditions.
These findings show that aging β cells are not irreversibly damaged. Instead, they can be functionally rejuvenated when key cellular signals are restored.

Stem Cell–Derived Vesicles Restore Mitochondrial Energy Production and Insulin Secretion
Insulin release depends heavily on mitochondria, the cell’s energy-producing structures. In aging β cells, mitochondrial function declines, leading to impaired insulin secretion and increased oxidative damage.
The researchers found that vesicle treatment restored mitochondrial activity, reduced harmful reactive oxygen species, and re-established normal energy production. As mitochondrial function recovered, β cells regained their ability to release insulin in response to glucose.
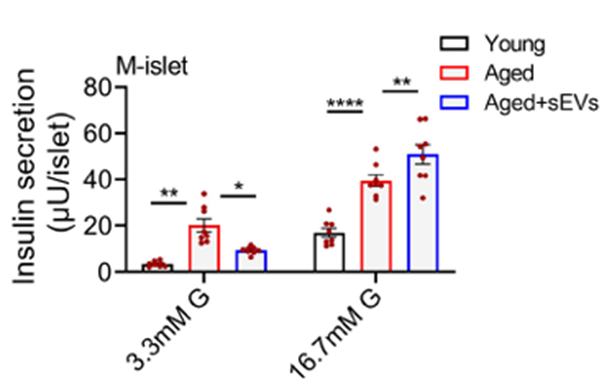
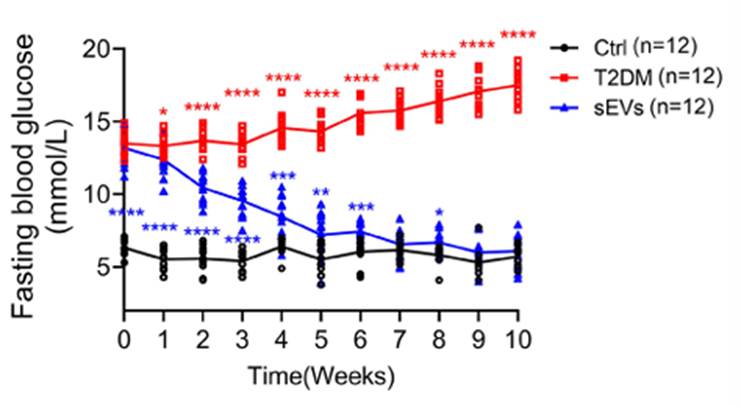
Stem Cell–Derived Vesicles Improve Glucose Control and Insulin Sensitivity in Aged Mice
Encouraged by the cellular results, the team tested the therapy in aged mice with diabetes induced by a high-fat diet and mild pancreatic injury. Mice receiving regular injections of the stem cell vesicles showed sustained reductions in blood sugar, improved glucose tolerance, and increased insulin levels compared with untreated diabetic animals.
Importantly, the treatment reduced the number of senescent β cells while preserving overall pancreatic structure. The mice also showed improved insulin sensitivity in peripheral tissues such as the liver and fat, suggesting systemic metabolic benefits.
A Single microRNA Drives the Rejuvenating Effects of Stem Cell–Derived Vesicles
The researchers next asked how the vesicles exerted their effects. Detailed molecular analysis revealed that the vesicles were rich in miR-21-5p, a small regulatory RNA that controls gene activity rather than coding for proteins.
In aging β cells, miR-21-5p levels decline. Restoring this RNA suppressed chronic inflammatory signaling driven by the IL-6 pathway, a major contributor to cellular aging. This, in turn, reactivated a key mitochondrial channel responsible for calcium handling, which is essential for insulin release.
When miR-21-5p was removed from the vesicles, most of the rejuvenating effects disappeared, confirming that this molecule is a central driver of the therapy’s benefits.
A Cell-Free Vesicle Therapy Targets Aging Without Stem Cell Transplantation
Unlike traditional stem cell therapies, this approach does not rely on transplanting living cells. Instead, it harnesses the signaling molecules naturally packaged into stem cell–derived vesicles. This avoids risks such as immune rejection or uncontrolled cell growth while retaining therapeutic potency.
The study demonstrates that targeting cellular aging directly, rather than simply lowering blood sugar, can reverse diabetes-related decline in old animals. While these findings are limited to mice, they provide a compelling framework for developing senescence-targeted therapies that preserve tissue function instead of eliminating cells.
Further studies will be needed to determine whether similar benefits can be achieved safely in humans. For now, the work highlights the possibility that age-related diabetes may be treated by restoring cellular youth, rather than compensating for its loss.
Model: Male C57BL/6 mice aged 18 months, fed a high-fat diet and given low-dose streptozotocin (STZ) to induce age-associated type 2 diabetes and pancreatic β-cell dysfunction.
Dosage: Human amniotic membrane mesenchymal stem cell–derived small extracellular vesicles (hAMSC-sEVs) at 1 mg/kg body weight (total vesicle protein), administered via tail vein injection in 200 µL PBS, twice weekly for 8 weeks (16 total injections).

