Study Shows New “Longevity Gene” Reverses Heart and Blood Vessel Function Decline
British and Italian scientists rejuvenate the aging heart by delivering the longevity gene LAV-BPIFB4 to human cells and mice.
Highlights:
- Aged human hearts have lower levels of LAV-BPIFB4 longevity gene activation, along with fewer small blood vessels and more senescent (aged) cells.
- Treating aged human heart cells with longevity protein LAV-BPIFB4 reduces senescent cell burden.
- Delivering the longevity gene via gene therapy methods (AAV9) restores blood vessels and increases blood flow in the hearts of mice.
If not genetically inclined, our hearts will deteriorate with age. This includes loss of heart contractility, increased artery stiffness, loss of small blood vessels (capillaries), and a reduced capacity to increase blood flow in response to stressors like exercise. However, those who live to the age of 95 and beyond — long-living individuals (LLIs) — are often free from this decline until their very last years.
Now, researchers may have found the gene responsible for delaying heart aging in LLIs. In a report published in Cardiovascular Research, Cattaneo and colleagues show that aged human hearts have less of the longevity protein (coded for by the longevity gene). Aged human hearts also have fewer capillaries — small blood vessels — and more senescent cells. Restoring the longevity protein reduces these senescent cells. Furthermore, viral-mediated delivery of the longevity gene reverses features of heart aging in mice.
Longevity Protein Reverses Heart Aging
Cattaneo and colleagues obtained biopsies from twenty-four patients undergoing heart transplantation for end-stage ischemic heart failure (IHF). These samples were compared to samples from younger individuals. IHF hearts showed lower levels of BPIFB4, fewer capillaries, and increased markers for senescent cells — cells that promote aging when accumulated over time. Supplementing IHF cells with the longevity protein LAV-BPIFB4 in a dish (in vitro) reduced these senescent markers, suggesting that the longevity protein can reverse features of heart aging.
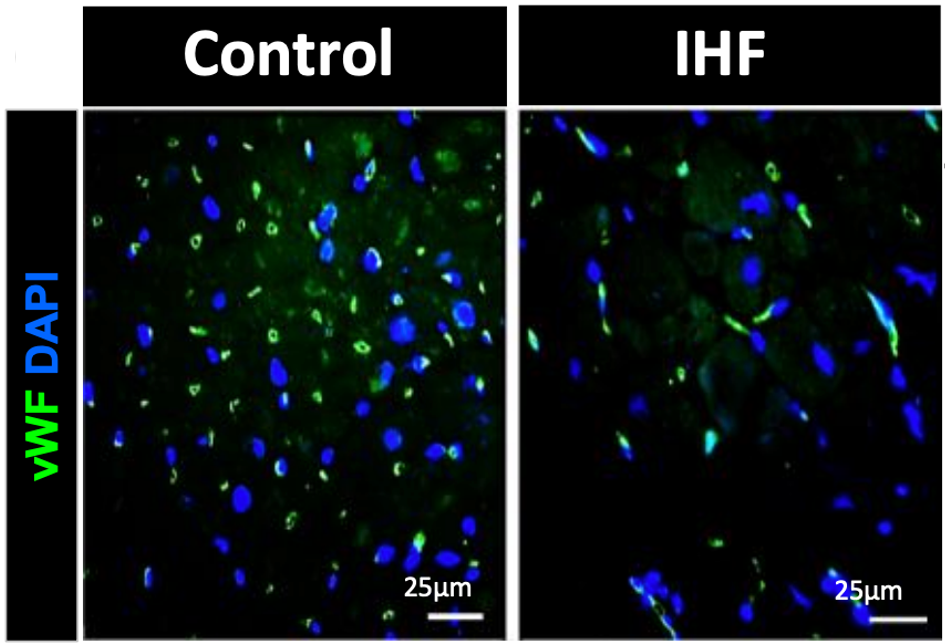
Longevity Gene Rejuvenates Aged Hearts in Mice
To determine the effect of activating the longevity gene via gene therapy, Cattaneo and colleagues injected 18-month-old elderly mice with the LAV-BPIFB4 gene for one month using an adeno-associated virus (AAV9) delivery system. This resulted in improved systolic function, increased capillaries, decreased senescent cells, and reduced scarring in the hearts of elderly mice, suggesting rejuvenation of the heart.
Furthermore, in both elderly and middle-aged mice (14 months old), gene therapy-mediated induction of LAV-BPIFB4 restored blood flow capacity in response to stress, important for cardiovascular fitness and health. This was measured by imaging the heart with PET in response to adrenergic stimulation, similar to an adrenaline rush.
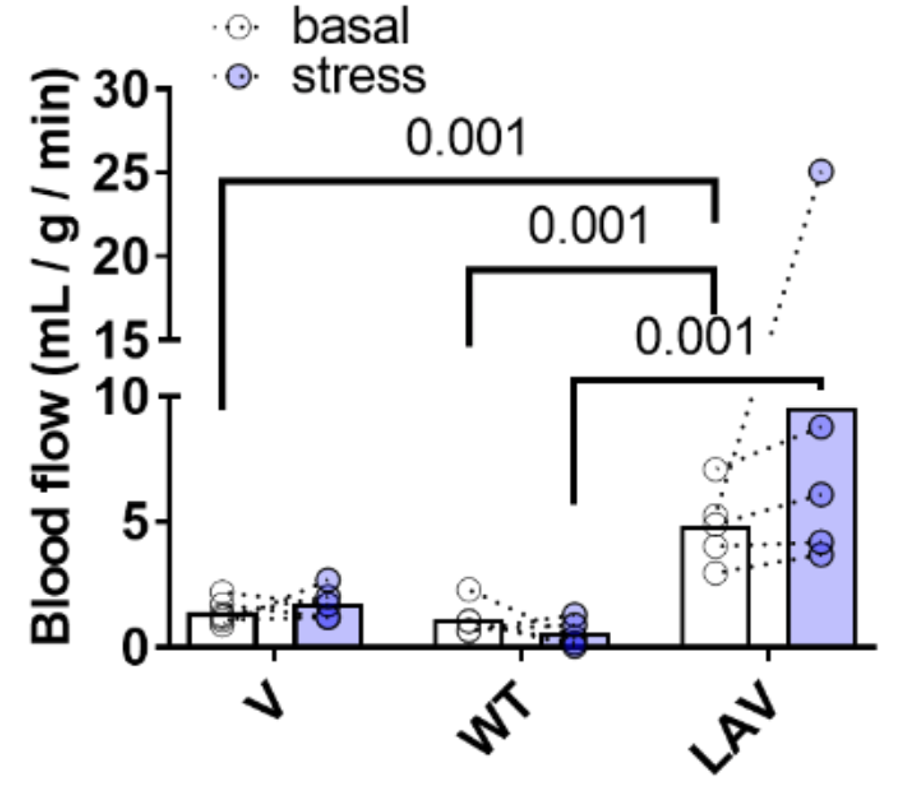
The findings of Cattaneo and colleagues suggest that we can potentially use gene therapy to promote longevity in humans with the longevity gene (which leads to the production of the longevity protein). They also found that a protein called nucleolin, which plays a critical role in lifespan extension, binds to the longevity protein. It is the binding of the longevity protein to nucleolin that may be responsible for increasing blood vessel formation and blood flow in aged hearts.
It was recently found that capillary cells called pericytes regulate blood flow. Interestingly, Cattaneo and colleagues found that pericytes are reduced in aging human and mouse hearts. Furthermore, LAV-BP1FB4 gene therapy restored the pericytes in older mice. Together, these findings point out the importance of capillary function and blood flow in aging.
The Longevity Gene and High NAD+
A previous study has shown that LLIs have higher levels of NAD+, which may help explain why they live so long. Therefore, it is possible that the longevity gene not only reduces senescent cells, which promote the chronic inflammation associated with aging but also boosts NAD+ levels. This is in addition to the longevity gene mediating increased activation of nucleolin, which also promotes longevity via promoting increased blood flow.
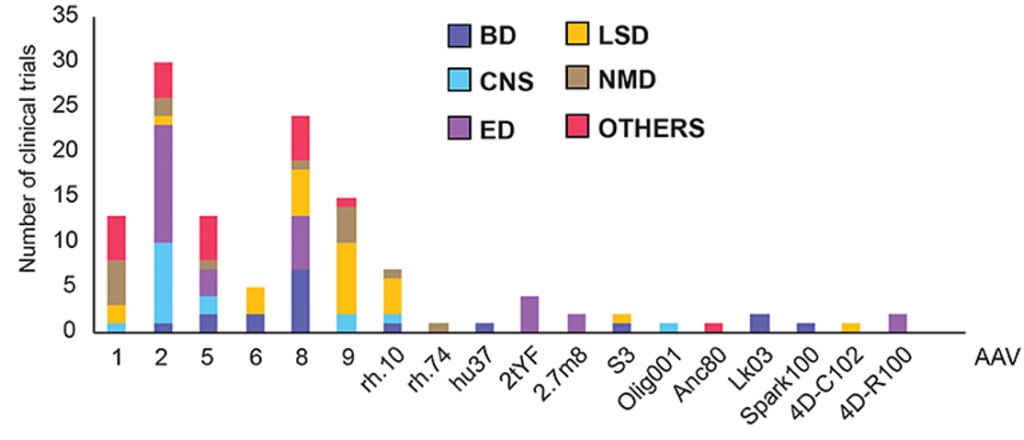
AAV-Mediated Gene Therapy
Cattaneo and colleagues utilized a viral vector called AAV9 to deliver the longevity gene to mice. AAV delivery vectors utilize the machinery used by viruses to infiltrate cells, but the virus is replaced by a gene of choice. AAVs, including AAV9, are already being tested in clinical trials, suggesting their feasibility for delivering the longevity gene to humans, although not many current AAV therapies target the heart.