Study Shows NMN Stops Blood Vessel Aging in Mice
NAD+ decline helps drive cardiovascular disease in mice, which can be mitigated with the precursor NMN
Highlights
· Deficiency of CD38 — an enzyme that consumes NAD+ — alleviates high blood pressure and cardiovascular disease-related blood vessel changes.
· NMN supplementation or CD38 inhibition restores cardiovascular function by ameliorating blood vessel cell cycle arrest (senescence).
CD38 is the main enzyme for the degradation of nicotinamide adenine dinucleotide (NAD+) — a cofactor in 400+ critical cellular activities — a process shown to contribute to aging. In their article published in Signal Transduction and Targeted Therapy, researchers from Sichuan University and Nanchang University show that CD38 and the associated intracellular NAD+ decline are critical for processes that drive age-related cardiovascular diseases, such as atherosclerosis and hypertension.
In mice lacking CD38 or supplemented with a CD38 inhibitor or the NAD+ precursor nicotinamide mononucleotide (NMN), the researchers blocked the development of cardiovascular diseases by limiting high blood pressure and increases in blood vessel cell senescence — a permanent state when cells can no longer replicate. In addition, the researchers observed that CD38 deficiency or NAD+ supplementation remarkably mitigated senescence of blood vessel cells by suppressing signaling payloads between cells called extracellular vesicles that facilitated the senescence of neighboring non-damaged cells.
“‘NAD-boosting’ therapy and the application of CD38 inhibitors may be novel strategies for treating age-related diseases,” proposed the researchers.
Senescence can drive cardiovascular disease
The incidence of cardiovascular diseases significantly increases with aging. The accumulation of cells that become senescent — a state of permanent cell cycle arrest — is a hallmark of aging and has been demonstrated to be involved in the pathological progression of cardiovascular diseases, including heart failure, atherosclerotic disorder, and hypertension. The senescence of vascular smooth muscle cells (VSMCs) contributes to pathological vascular remodeling and the deterioration of hypertension control.
NAD+ decline drives senescence of blood vessel cells
This study showed that genetically removing CD38 or blocking CD38 with an inhibitor significantly alleviated chemically-induced vascular remodeling in mice, a model for cardiovascular disease development. When the researchers genetically deleted CD38 from mice, these animals had improvements in processes that drive cardiovascular diseases, such as high blood pressure (hypertension), increased thickness of the blood vessel wall (indicative of stiffer and less functional blood vessels) , and alterations in the level of proteins critical for maintaining blood vessel integrity.
Their results also showed that CD38 deficiency significantly attenuated chemically-induced DNA damage and reduced the accumulation of senescence-associated markers in VSMCs in the arteries of these mice.
“In the current study, we demonstrated that CD38 not only contributed to vascular remodeling in hypertension but also played a detrimental pro-senescence role in VSMC senescence, further confirming the correlation of NAD decline and aging,” concluded the researchers.
Boosting NAD+ levels alleviates hypertension and vascular remodeling
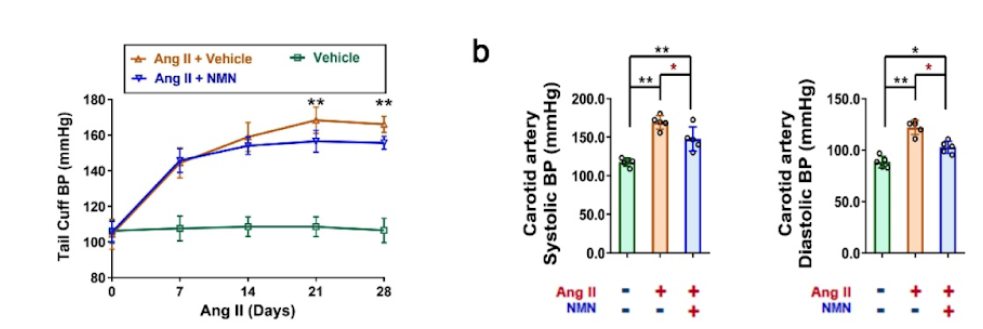
The researchers also examined whether NAD+ supplementation can rescue VSMC senescence. Under healthy conditions, the intracellular NAD+ levels of whole aorta tissue were increased by nearly 50% in mice lacking CD38 compared with unmodified mice. Moreover, oral administration of NMN (300 mg/kg) to elevate NAD+ levels alleviated chemically-induced hypertension. In these hypertensive mice, the vascular media thickness, media-to-lumen ratio, and collagen deposition were reduced by 26%, 27%, and 30%, respectively, following injection with NMN (i.p., 10 mg/kg/dose).
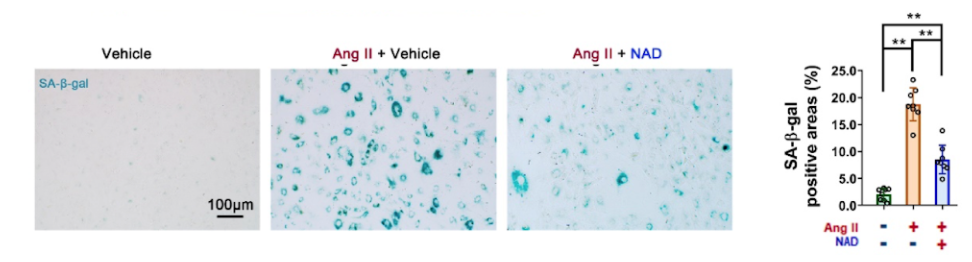
In cell culture, the researchers pre-administered exogenous NAD+ (100 μM) to VSMCs and detected Ang II-induced cell senescence indicators. The results showed that the area positive for senescent cells was decreased by 54%. In addition, when the researchers increased the levels of CD38, the levels of senescence of VSMCs significantly increased. These results strongly supported that CD38 may be a potential pharmacological target for anti-senescence treatment.
CD38 and NAD+ control intercellular cargo containers that induce senescence
The researchers demonstrated that CD38 deficiency reduced VSMC senescence by suppressing the genesis, secretion, and internalization of extracellular vesicles — spherical containers made of the same fat that encases cells. These structures can deliver molecules between cells and organs, including molecules that promote neighboring cell senescence in aging processes.
In this study, small extracellular vesicles derived from Ang II-challenged smooth muscle cells accelerated the senescence of neighboring undamaged cells. Moreover, these small extracellular vesicles were easily internalized by senescent cells, exacerbating the aging process in these cells.
“Accordingly, these results advance our comprehension of the mechanisms of VSMC senescence in hypertension and provide insight into novel therapeutic targets for reducing vascular aging,” concluded the authors. “Our results provide strong evidence that CD38 inhibition or NAD supplementation may serve as potential therapeutic strategies for age-related diseases.”

