Study Suggests Rapamycin Can Overcome Genetic Differences in Vascular Aging
Scientists find that the longevity-associated drug rapamycin alleviates vascular aging in rats with low physical endurance.
Highlights:
- Old rats with low running capacity have increased artery stiffness — the arterial hardening associated with heart disease and early death.
- The immunosuppresent drug rapamycin can rescue mitochondrial impairment in artery cells from old rats with low running capacity.
Our capacity for endurance exercise depends largely on our cells’ ability to utilize oxygen. Older adults with low endurance capacity and poor oxygen utilization are at higher risk for premature death. Moreover, rats with low endurance capacity live 28-45% shorter lives. With age, our endurance capacity declines due to factors like stiffening arteries and impaired artery cell mitochondria. Now, a new study suggests that this age-related vascular decline is genetic and can be mitigated with a common immunosuppressant.
As reported in the Journal of Cardiovascular Aging, researchers from the University of Michigan selectively bred rats for low or high inborn running capacity. They found that only old low-capacity runners (LCR) exhibit artery stiffness and mitochondrial impairments, while old high-capacity runners (HCR) have similar vascular health to young rats. Furthermore, rapamycin was shown to enhance mitochondrial health in old LCR rats, suggesting that rapamycin can mitigate genetic differences in endurance capacity.
Only Low-Capacity Runners Exhibit Age-Related Vascular Dysfunction
To separate LCR from HCR rats, Canugovi and colleagues measured the distance lab rats could run on a treadmill before exhaustion. The rats that ran the farthest (top 20%) were bred for their physical fitness traits and placed into the HCR group. Similarly, the rats that ran the shortest distances (bottom 20%) were bred to make up the LCR group.
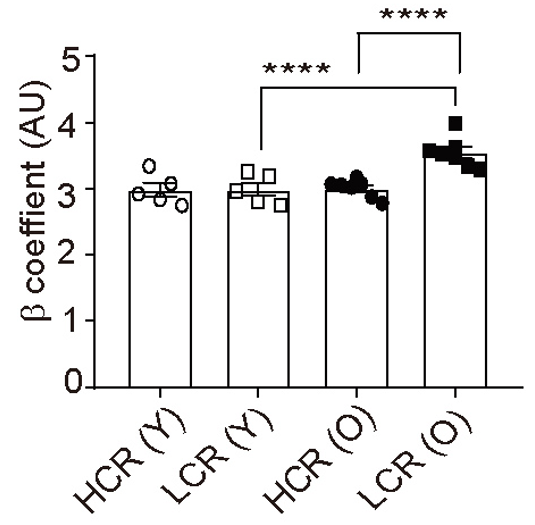
Artery stiffness was calculated from changes in artery diameter in response to changes in pressure, similar to the changes in diameter that would occur in response to blood pressure changes. Compared to young LCR rats, old LCR rats had increased artery stiffness. Furthermore, old HCR rats had levels of artery stiffness similar to the young rats. These findings suggest that artery stiffness is the result of genetic traits and not necessarily age.
Mitochondrial impairment may underlie arterial hardening, which leads to cardiovascular disease (CVD) and subsequent high mortality risks. Usually, our cells selectively clear damaged mitochondria through a process called mitophagy. However, with aging, mitophagy declines. A lack of mitophagy and more damaged mitochondria promotes oxidative stress — damage caused to cells by harmful molecules called reactive oxygen species — and inflammation, both hallmarks of aging.
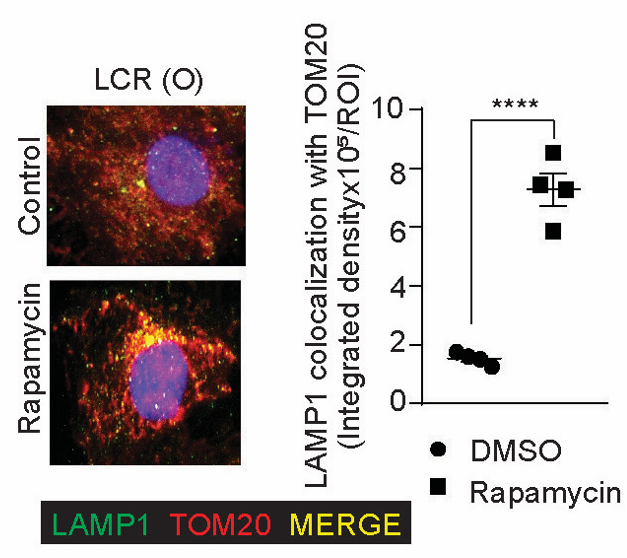
Canugovi and colleagues observed a 76% reduction in mitophagy from the muscle artery cells (vascular smooth muscle cells) of old LCR rats, along with increased oxidative stress and inflammation. Rapamycin, a well-known mitophagy inducer that reduces artery stiffness, was shown to increase mitophagy in these artery cells and decrease oxidative stress and inflammation. Furthermore, rapamycin treatment increased the mitochondrial oxygen consumption rate, demonstrating an enhancement of oxygen utilization.
Is Taking Rapamycin as an Anti-Aging Agent a Good Idea?
Rapamycin is a prescription medication used to prevent the immune system rejection of organ transplants. It is also an inhibitor of mTOR. Since inhibiting mTOR is associated with increased lifespan, individuals may wish to use rapamycin as an anti-aging therapeutic. At the correct dose, rapamycin has a similar range of side effects as over the counter drugs like aspirin and may be safe.
The findings of Canugove and colleagues suggests that genetics contribute largely to vascular aging and that rapamycin partially overcomes these genetic differences. From this, the question is whether rapamycin is necessary for all. Could those not genetically susceptible to vascular aging benefit from rapamycin?
It may be possible that only some of us with specific genetic traits can benefit from the anti-vascular aging effects of rapamycin. Some of us may have genes more conducive to physical fitness, rendering rapamycin ineffective. For example, a recent study found a human gene associated with increased skeletal muscle oxygen utilization and cardiorespiratory fitness. Individuals with this gene and the like may not need rapamycin. Potentially, many anti-aging interventions may only be necessary for individuals with specific genetic traits, giving heed to personalized medicine — using an individual’s genetic profile to make treatment decisions.

