Supplementing NAD+ Curtails Zika-Related Birth Defects, Promoting Newborn Mice Survival
New research points to exploiting NAD+ levels to develop therapeutic strategies against the Zika virus and other mosquito-borne diseases.
Highlights
· To restore NAD+ levels altered by the Zika virus, Hu and colleagues injected NAD+ or NR to preserve neurons, maintain brain weight, and improve survival.
· The study suggests that boosting NAD+ levels in newborns infected with the Zika virus can slow or even prevent the virus’s damaging effects.
Not only are mosquitos pesky and annoying, but they have also affected innumerable lives by transmitting pernicious diseases. One of the most recent mosquito-borne diseases comes from the Zika virus, linked to drastic head size reductions (microcephaly) in newborns of pregnant women. Finding new ways to counter the handicapping effects of the virus has become paramount to preserve these newborns’ quality of life.
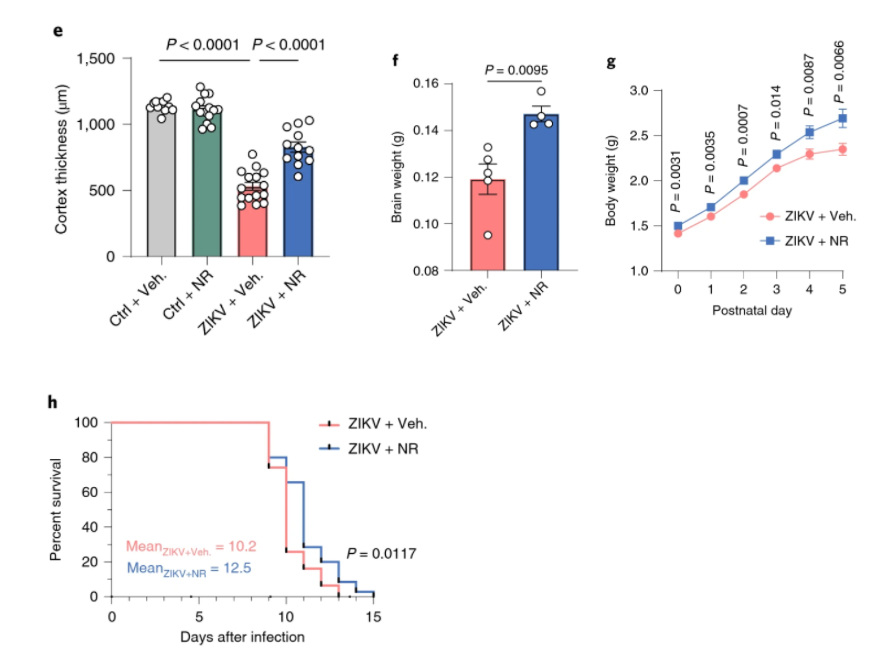
New research from Hu and colleagues published in Nature Metabolism demonstrated profoundly abnormal nicotinamide adenine dinucleotide (NAD+) metabolism with Zika infection in newborn mice. The Tsinghua University-based research team then boosted NAD+ levels by injecting the molecule or its precursor nicotinamide riboside (NR) in Zika-infected mouse pups. By increasing NAD+ levels, these treatments preserved neurons, brain weight, and improved overall survival for the infected mouse pups. Hu and colleagues’ study shows that the Zika virus dramatically alters NAD+ metabolism and that increasing its levels can counter the virus’s microcephaly-inducing effects.
Viruses Reprogram Host Metabolism
Viruses reprogram infected host metabolism through many unique strategies specific for each virus to achieve rapid proliferation. With that in mind, Hu and colleagues wanted to uncover how Zika reprograms its host cells so they could target these metabolic changes with antiviral therapy. The Chinese researchers examined gene activity, protein production, and metabolite profiles of Zika-infected mouse pups to uncover how the Zika virus reprograms the host cells.
Their gene activity analysis showed that infecting the embryonic mice increased the abundance of brain immune cells that dispose of the dead, dying, injured, or diseased neurons. What’s more, this analysis of gene activity also indicated a reduced abundance of cells that produce neurons along with reduced numbers of neurons, which aligns with Zika infections driving reduced brain weights and volumes. Similarly, their protein abundance analyses showed reduced levels of proteins associated with metabolic reactions that involve NAD+. This finding hinted that NAD+ might play a crucial role in Zika virus-induced microcephaly.
In search of evidence that Zika reprograms NAD+ metabolism and that targeting NAD+ might provide a way to minimize microcephaly, Hu and colleagues looked at the molecule’s levels in the brain. They found that the virus substantially reduces NAD+ abundance in mouse pup brains while increasing levels of its precursors nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR). These findings came from altered NAD+ metabolism that goes hand-in-hand with a greater abundance of NAD+-consuming proteins.
Increasing NAD+ Levels Preserves the Newborn Mouse Brain with Zika Infection
Since Hu and colleagues saw that NAD+-consuming proteins become more abundant and deplete NAD+ with Zika infection, they decided to test whether boosting NAD+ can preserve the brain. They found that increasing NAD+ levels by injecting NAD+ or its precursor NR maintained brain weight and volume in infected mouse pups, and NR also helped their survival. These findings provide compelling evidence that NAD+ metabolism represents a targetable pathway to counter the Zika virus’s effects in newborns, at least in mice.
The study supports that measuring gene activity, protein abundance, and metabolite levels can provide important insight for targetable disease pathways. This useful information can spawn the production of new therapies to hinder or altogether prevent disease progression.
Increasing NAD+ May Blunt Zika’s Effects in Human Newborns
In the case of mice, boosting NAD+ levels with precursor supplementation preserves neurons and brain integrity. The translatability of these findings to humans may provide an important means to prevent severe microcephaly in newborns with Zika-infected mothers. The most important aspect of using NAD+ precursors to preserve brain tissue in newborns is to test mothers more frequently for the infection since adults often show no symptoms.
The findings from this study suggest that restoring NAD+ levels with NAD+ or precursor injections can ameliorate the progression of Zika virus effects. Yet, it’s possible that boosting NAD+ levels during other types of mosquito-borne viral infections can enhance the body’s response to their damaging effects as well.
Interestingly, Hu and colleagues’ data shows that NR increased levels of the immediate NAD+ precursor NMN. This finding raises the possibility that perhaps direct supplementation with NMN would enhance NAD+ levels more robustly and provide more protection from the Zika virus in newborns. To get the most significant amount of protection from Zika virus, it would be beneficial for future studies to compare the anti-viral effects of the NAD+ precursors NMN and NR.

