Taurine Reverses Pancreatic Aging, Offering Hope for Type 2 Diabetes
New study shows that prostaglandin E₂ (PGE₂) can restore strength and regeneration in aged muscle by rebooting stem cell function.
Highlights:
- Daily taurine supplementation reversed β-cell senescence and restored insulin secretion in pancreatic cells from aged mice.
- Taurine also shielded β-cells from inflammation and DNA damage without triggering cell death.
As we age, our ability to regulate blood sugar quietly falters—not because we run out of insulin, but because the cells that produce it grow tired, inflamed, and eventually fall silent. These cells, known as pancreatic β-cells, are responsible for making the insulin hormone that keeps glucose levels in check. But over time, they accumulate stress and damage, entering a state of cellular senescence—a dysfunctional “zombie” mode where the cells stop dividing and start spreading inflammatory signals to their neighbors.
This senescent shift in β-cells plays a central role in the development and progression of type 2 diabetes, particularly in older adults. But what if we could reverse it?
A new study suggests we might.
Testing Taurine’s Anti-Aging Effects on Pancreatic Cells
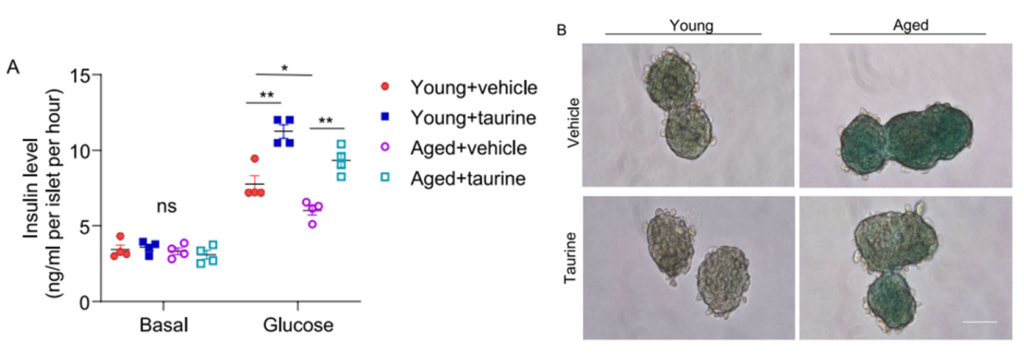
To explore taurine’s potential, researchers first isolated pancreatic islets—clusters of hormone-producing cells in the pancreas—from both young and aged mice. These islets contain the β-cells responsible for producing insulin. As expected, the islets from aged mice showed clear signs of decline: they secreted less insulin in response to glucose and showed elevated levels of β-galactosidase, an enzyme often used as a marker for cellular senescence (the state in which cells permanently stop dividing and begin secreting inflammatory signals).
When the aged islets were treated with physiological levels of taurine (100 µM), insulin secretion was restored to more youthful levels. At the same time, the number of β-gal-positive (senescent) cells decreased, suggesting that taurine was reversing the senescent state or at least alleviating its harmful effects.
Fighting DNA Damage and Inflammation Without Triggering Cell Death
To mimic one of the key drivers of aging—DNA damage—the team exposed cultured mouse β-cells (MIN6 cells) to doxorubicin, a chemotherapy agent known to break DNA strands. As expected, the damaged cells showed a spike in γH2AX, a marker of DNA damage, and began producing high levels of senescence-associated proteins such as p53, p21 (Cdkn1a), p16 (Cdkn2a), and inflammatory signals like Gdf15, Ccl2, and IL-1β.
Yet when the cells were pre-treated with taurine, this entire senescent cascade was suppressed. The cells showed fewer signs of DNA damage, reduced expression of senescence markers, and a marked decline in pro-inflammatory secretions—collectively known as the senescence-associated secretory phenotype (SASP). Notably, this occurred without triggering cell death: levels of apoptosis-related genes like Fas and Bax remained unchanged, and there was no increase in overall cell death measured by flow cytometry.
This distinction is critical. Many drugs that remove senescent cells—so-called senolytics—do so by selectively killing them, which can carry risks in tissues with limited regenerative capacity. Taurine, by contrast, appears to rejuvenate β-cells without destroying them.
How Taurine Protects Cells from Inflammatory Damage
Cellular aging isn’t always caused by DNA damage. Chronic inflammation, a hallmark of aging, can also drive senescence. To test taurine’s resilience under inflammatory stress, researchers exposed MIN6 cells to TNF-α, a pro-inflammatory cytokine elevated in aging tissues.
Once again, TNF-α triggered increased expression of senescence and SASP markers like p53, p21, Gdf15, Ccl2, and IL-1β. And once again, taurine pre-treatment dampened this inflammatory cascade without activating apoptotic genes.
These findings suggest that taurine’s anti-senescent effects are not limited to one aging pathway. Whether β-cells are pushed into senescence by damaged DNA or inflammatory signaling, taurine helps restore a more youthful gene expression profile.
A Natural Molecule with Therapeutic Potential
Taurine is an amino acid compound naturally found in meat, fish, and dairy, and is commonly added to energy drinks. Previous studies have linked taurine to cardiometabolic health, but its role in aging and cellular senescence has only recently gained attention. A 2023 study in Science found that taurine levels decline with age in multiple species and that supplementation extended lifespan in mice; however, some studies have shown that taurine is elevated in older adults.
This new study builds on those findings by showing that taurine may specifically target pancreatic β-cell aging, a major contributor to diabetes progression. By reducing senescent cell burden, restoring insulin production, and lowering inflammation, taurine emerges as a promising candidate for treating age-related metabolic diseases.
Taurine and the Future of Anti-Aging Therapies
While this study offers compelling evidence that taurine can reverse β-cell senescence and restore insulin function in diabetic mice, further research is needed to evaluate its efficacy in human tissues and confirm long-term safety. The precise mechanisms behind taurine’s anti-senescent effects—whether through direct clearance of senescent cells, suppression of SASP factors, or modulation of gene expression—remain to be fully elucidated.
Future investigations should explore taurine’s potential synergy with existing diabetes treatments or other senolytic compounds. As a naturally occurring molecule with a strong safety profile, taurine serves as a promising, low-risk candidate for interventions targeting both metabolic dysfunction and cellular aging. For individuals with type 2 diabetes or at risk of age-related β-cell decline, taurine could represent an accessible and innovative strategy to preserve pancreatic health and slow disease progression.

