The Sirtuin Secret: New Study Shows How NMN Helps These Longevity Enzymes Combat Aging
Study confirms that sirtuin enzymes, major regulators of cellular aging, mediate many of the beneficial effects of NMN on the brain and gut of aged mice.
Highlights
- NMN reduces brain inflammation and improves memory by activating sirtuins.
- NMN improves gut barrier integrity and protective lining by activating sirtuins.
- NMN-activated sirtuins reduce underlying biological drivers of aging, including inflammation and senescent cells.
In the quest to understand and potentially slow the aging process, scientists have increasingly focused on a family of enzymes called sirtuins. These remarkable regulators, particularly one known as Sirtuin 1 (Sirt1), are emerging as key players against age-related diseases. A new study published in Frontiers in Pharmacology reveals how Sirt1 mediates NMN’s effects against neurodegeneration and gut barrier aging, offering hope for conditions ranging from Alzheimer’s disease to inflammatory bowel disorders.
The Sirtuin Switchboard of Aging
Sirtuins are like molecular switchboards that coordinate countless processes essential for cellular health and longevity. These specialized proteins function as NAD+-dependent deacetylases—enzymes that remove acetyl groups from other proteins, effectively changing how those proteins function. They regulate everything from DNA repair and energy metabolism to inflammation and cellular stress responses. As we age, however, sirtuin activity naturally declines.
What makes sirtuins particularly interesting is their dependence on NAD+ (nicotinamide adenine dinucleotide). This vital coenzyme serves as the “fuel” that powers sirtuin activity. Unfortunately, NAD+ levels naturally decline with age, dropping by up to 50% between youth and old age in mammals. Thus, not only do we produce fewer sirtuins as we age, but we also have less of the NAD+ fuel they need to function properly. The relationship between NAD+ and sirtuins has sparked intense scientific interest in NAD+ precursors—compounds that can boost NAD+ levels in the body. One such precursor is NMN (nicotinamide mononucleotide), the focus of the recent study.
NMN-Activated Sirtuins Counteract Brain Aging
In the new study, conducted by researchers in China, mice were injected with a sugar called D-galactose (D-gal) to accelerate their aging. These mice typically develop symptoms remarkably similar to human aging: cognitive decline, increased inflammation, and even intestinal barrier dysfunction.
The effects of feeding NMN to these prematurely aging mice were striking. The treatment significantly reduced brain inflammation and oxidative stress—damage caused to cells by oxidizing free radicals called reactive oxygen species (ROS). NMN also improved locomotor activity, as indicated by the mice moving more in an open field, and cognitive function, as shown by a common method known as the Morris Water Maze test. Most importantly, all these benefits disappeared when the researchers also administered a Sirt1 inhibitor, confirming that the Sirt1 pathway was responsible for the improvements.
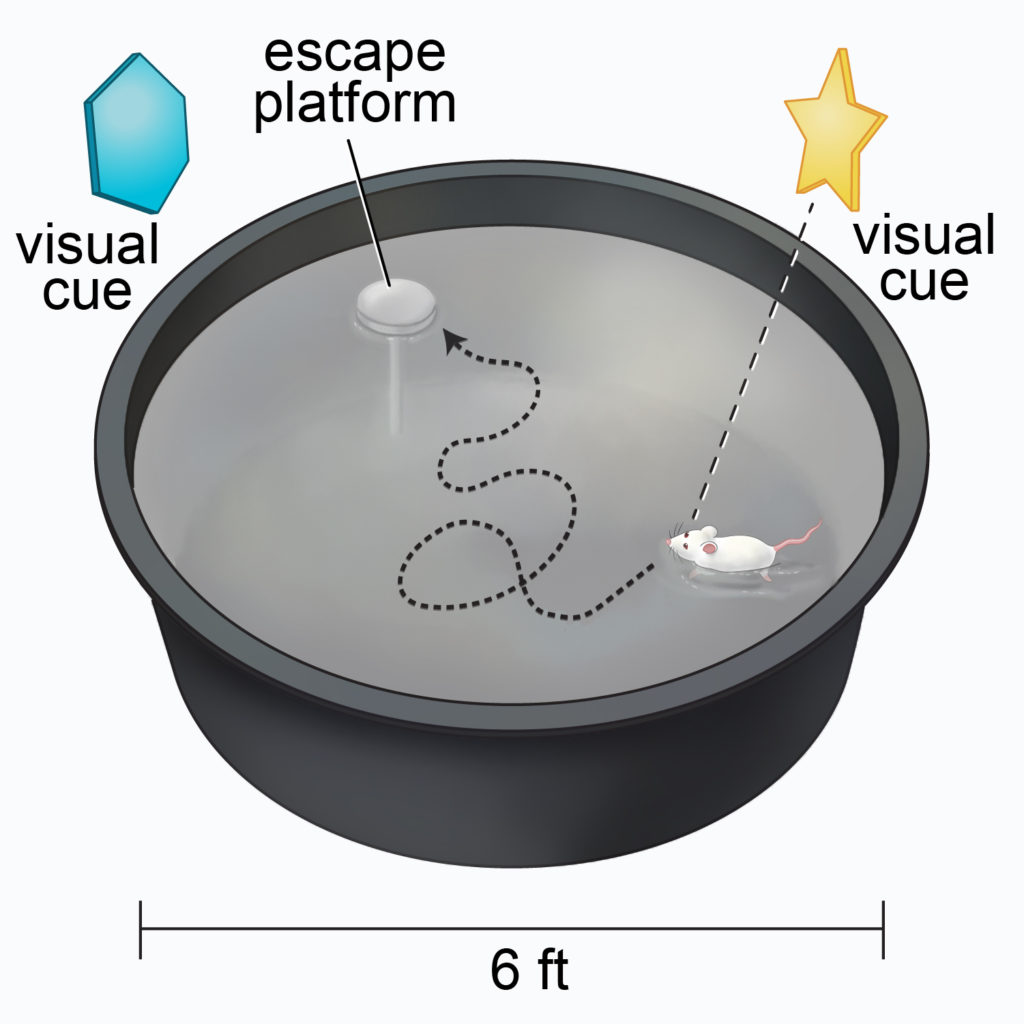
The Morris Water Maze involves a mouse being placed into a pool of water containing an escape platform. Visual cues surround the pool to help the mouse find the platform and escape from drowning. The mouse learns the location of the platform, which is always placed in the same quadrant of the pool, over two or three consecutive training days. On the test day, the researchers remove the platform and record how often the mouse crosses, moves around, and spends time in the correct quadrant. More time spent in the target quadrant is interpreted as having better memory.
The researchers showed that the age-accelerated mice crossed the target quadrant far less than normal mice. The age-accelerated mice also moved around less and spent less time in the target quadrant, suggesting impaired memory. Interestingly, feeding the age-accelerated mice NMN did not significantly increase the number of crossings or time spent in the correct quadrant. However, it did significantly increase distance moved, an effect that disappeared by inhibiting Sirt1 with a compound called Ex527.
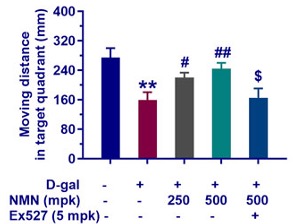
Considering that NMN only increased the distance traveled within the target quadrant and not the number of crossings or time spent in the correct quadrant, more studies may be needed to confirm whether NMN improves the memory of the researchers’ age-accelerated mouse model. With that being said, NMN was previously shown to significantly increase target quadrant crossings in D-gal age-accelerated mice.
NMN-Activated Sirtuins Counteract Gut Aging
One of the most intriguing findings from the study was how NMN-mediated Sirt1 activation improved intestinal health. Aging typically leads to a leaky gut barrier, a condition increasingly linked to systemic inflammation and even neurodegeneration. NMN treatment preserved the structural integrity of the intestinal lining and maintained the number of goblet cells, which produce a protective mucus. It also reduced inflammatory cell infiltration and promoted recovery of both crypt depth and villus height—all indicators of a healthy intestinal barrier.
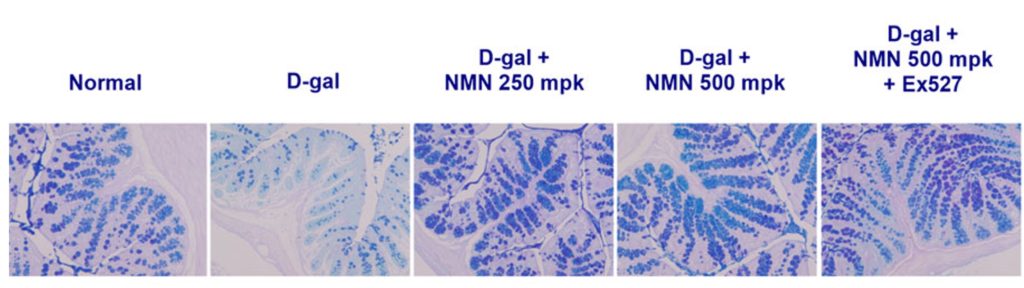
The gut-brain axis is bidirectional. Intestinal barrier dysfunction allows bacterial endotoxins to enter the bloodstream, triggering systemic inflammation that can affect the brain. By strengthening the gut barrier through Sirt1 activation, NMN may be able to reduce neuroinflammation and potentially slow neurodegenerative processes.
Of particular interest is how Sirt1 activation simultaneously protects both the brain and gut, highlighting the gut-brain axis. The results also emphasize the applicability of elevating NAD+ and activating sirtuins in multiple organs. While the researchers did not examine other organ systems, previous studies have shown that NMN elevates NAD+ levels in organs like the heart, fat tissue, and bone. Restoring NAD+ levels to these organs protects against conditions of aging, including cardiovascular disease, metabolic syndrome, and osteoporosis.
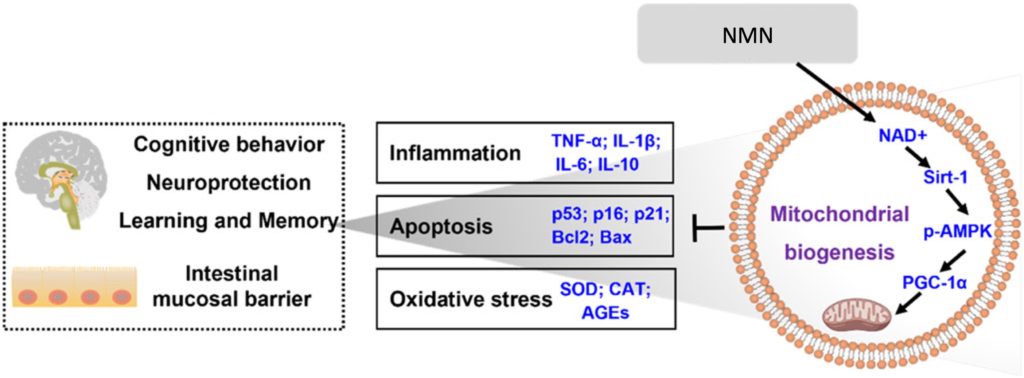
The Sirtuin Signaling Cascade: How It All Works
The study revealed that NMN’s beneficial effects stem from its ability to activate a specific molecular pathway: Sirt1/AMPK/PGC-1α. When NAD+ levels increase thanks to NMN supplementation, Sirt1 becomes more active. This, in turn, activates an enzyme called AMPK (adenosine monophosphate-activated protein kinase), which then increases the activity of PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha). The researchers showed that NMN stimulated this molecular cascade to produce the following beneficial effects:
- Enhanced mitochondrial health: More efficient cellular energy production
- Reduced oxidative stress: Better management of harmful free radicals
- Decreased inflammation: Lower levels of inflammatory molecules like TNF-α and IL-6
- Suppressed cellular senescence: Fewer senescent cells, which accumulate and promote aging
- Reduced apoptosis: Less programmed cell death in healthy neurons
Combining Sirtuin Activators with Other Longevity Interventions
Previous studies have demonstrated that the effects of NMN and other NAD+ precursors are mediated by the activation of sirtuin enzymes. For example, in a sepsis (body-wide inflammation) mouse model, NMN was shown to boost cognition and reduce inflammation unless Sirt1 was blocked. While sirtuins use NAD+ as fuel, they can also be activated by other molecules. For instance, NMN enhanced the effect of a sirtuin activator called E1231 against metabolic syndrome.
Combining sirtuin activators, like NAD+ precursors and E1231, with other longevity interventions may provide a broader effect against the multifaceted nature of cellular aging by targeting many biological drivers of aging (e.g., inflammation, mitochondrial dysfunction, senescent cells). For example, lycopene, a potent antioxidant found in tomatoes, enhances NMN’s ability to improve memory in age-accelerated D-gal rats. Moreover, combining NMN with prebiotics and fiber may provide a synergistic effect against Alzheimer’s dementia.
In a clinical trial, a cocktail of compounds including the NAD+ precursor NR (nicotinamide riboside) was shown to reverse neurodegeneration and improve mitochondrial function in Alzheimer’s patients. This trial supports the applicability of combining sirtuin activators with other longevity interventions to target aging, particularly brain aging, in humans. Some companies, such as Seragon Biopharmaceuticals, have already applied the knowledge of combined longevity interventions to create Restorin, which contains longevity interventions that target the multifaceted nature of cellular aging.
Model: Male C57BL/6 mice treated with D-galactose to trigger accelerated aging
Dosage: 250 mg/kg or 500 mg/kg of NMN administered orally for 8 weeks

