Umbilical Cord Stem Cells Restore Brain Function and Reverse Age-Related Cognitive Decline: New Study Shows
Umbilical cord stem cells restore brain function in aged mice by correcting microglial metabolism, reducing inflammation, and improving memory performance.
Highlights
• Umbilical cord–derived stem cells restore memory performance in aged mice and reduce aging markers in the hippocampus, the brain region that supports learning and long-term memory.
• The treatment corrects a metabolic cycle inside aging immune cells, lowering inflammation and clearing excess fats that interfere with microglial function as the brain gets older.
A new study published in Aging Cell by Liang and colleagues reveals a striking way in which human umbilical cord–derived mesenchymal stem cells, or hUC-MSCs, can help restore the aging brain. The researchers found that these stem cells rejuvenate microglia, which are the brain’s resident immune cells, and in doing so, reverse age-related memory loss in mice. The recovery was linked to changes in a molecular system that connects inflammation with how cells process fats, known as the NF-κB–SREBP1 pathway.
Aging Microglia Lose Their Balance: Stem Cells Restore It
Microglia act as the brain’s maintenance crew, responsible for clearing away debris and damaged cells. As they age, they begin to accumulate tiny fat-filled compartments inside their cytoplasm known as lipid droplets. This buildup causes them to lose efficiency and become inflamed, creating a type of dysfunctional cell known as lipid droplet–accumulating microglia.
The researchers observed that in older mice, these microglia showed high levels of aging-related proteins such as p16 and p21 and produced excessive inflammatory molecules, including interleukin-6 and tumor necrosis factor alpha. These changes coincided with measurable cognitive decline. After four weeks of treatment with hUC-MSCs, the mice showed near-youthful memory performance and a marked reduction in aging markers within the hippocampus, which is the part of the brain that controls memory and spatial learning.
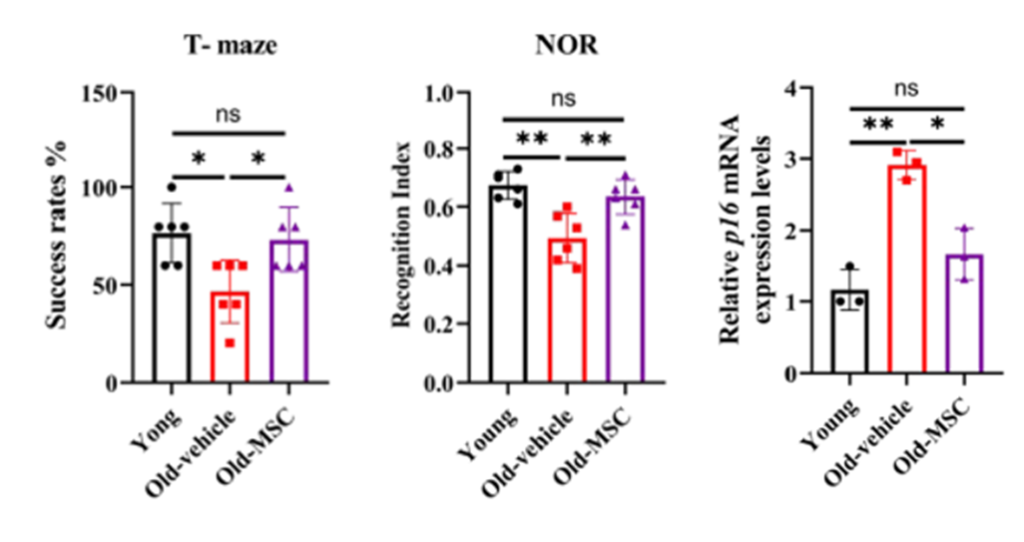
Stem Cells Disrupt the Feedback Loop Between Fat and Inflammation
The researchers found that the brain’s recovery could be traced to a disrupted chain reaction inside aging cells. Normally, two molecular regulators called NF-κB and SREBP1 become overactive as microglia grow older. NF-κB promotes inflammation, and SREBP1 controls how cells make and store fats.
When these two signals stay overactive for too long, they trap microglia in a harmful cycle where inflammation causes fat buildup, and that buildup keeps inflammation alive. Treatment with hUC-MSCs broke this cycle by quieting the signals that tell microglia to remain inflamed and overproduce fats.
Once the cells regained balance, they returned to their normal role of clearing waste and protecting neurons. This shift effectively reawakened the brain’s natural cleanup system and restored healthier communication between its immune and nerve cells.
In the Lab, Stem Cells Revived Senescent Microglia
To understand how this process worked inside cells, the researchers recreated it in the lab using microglia that had been artificially aged through exposure to hydrogen peroxide, a compound that causes oxidative stress. They then placed these aged microglia in a shared environment with hUC-MSCs, separated by a thin membrane that allowed only chemical signals to pass between them.
After several days, the aged microglia began showing signs of recovery. Their levels of a common aging marker called beta-galactosidase decreased, and the fat droplets that had built up inside them were greatly reduced. Most importantly, the cells regained their ability to clear waste from their surroundings, suggesting that the stem cells had helped restore their normal function.
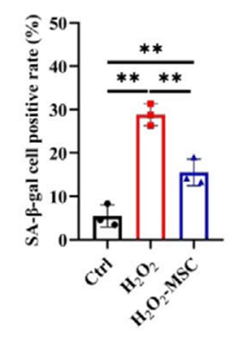
Blocking the Same Pathway Replicates the Effect
To confirm that the effect was linked to the same molecular pathway, the researchers treated the aged microglia with a small compound known to inhibit NF-κB activity. The results closely matched those seen with the stem cell treatment. The cells showed fewer signs of aging, less fat accumulation, and stronger cleanup activity.
This finding suggested that the benefit of hUC-MSCs did not come from general rejuvenation but from a specific correction of the inflammatory and fat-regulating pathway that drives microglial aging.
Rejuvenating Microglia May Help Protect Neurons From Degeneration
The study highlights how aging in the brain may stem less from widespread neuron loss and more from the gradual breakdown of its immune balance. Microglia, once thought of as passive support cells, have increasingly been recognized as active regulators of brain aging. When their metabolism shifts toward fat storage and chronic inflammation, the entire neural environment begins to deteriorate. The hUC-MSC treatment reversed that shift, restoring a metabolic profile more typical of youth and renewing the dialogue between immune and nerve cells.
Gene analysis of the hippocampus showed that this cellular renewal translated into broader molecular harmony. Pathways linked to immune regulation and lipid metabolism began to mirror those of younger brains, suggesting that the treatment reprogrammed how brain cells communicate rather than simply masking symptoms of aging.
Advancing a More Precise Approach to Brain Rejuvenation
This work adds to a growing scientific effort to improve brain aging through the correction of the metabolic weaknesses that develop inside immune cells. Several areas of longevity research are approaching this same problem from different angles. Senolytic therapies aim to remove cells that continuously release inflammatory signals, and calorie-restriction mimetics attempt to redirect energy metabolism toward more resilient programs. The findings from Liang and colleagues fit within this broader movement because the microglia functioned more effectively once their fat processing and inflammatory activity were brought back under control.
The results also connect with ongoing research on lipid metabolism in neurodegenerative disease. In many Alzheimer’s models, microglia accumulate fats they cannot break down, which prevents them from clearing damaged proteins and allows toxic material to build up. The improvement seen in the treated mice supports the idea that restoring healthy fat handling inside microglia can help maintain cleaner and more stable neural circuits.
The study offers a practical direction for future therapies. Researchers are already investigating the small vesicles and proteins released by stem cells to understand which specific signals trigger the metabolic corrections observed in this work. A refined understanding of these molecular cues may make it possible to design treatments that guide aging immune cells back toward healthier function without the need for full stem cell transplantation.
Model: Male C57BL/6 mice aged to 18 months to model natural brain aging and age-related cognitive decline.
Dosage: Human umbilical cord–derived mesenchymal stem cells at 1×10⁶ cells in 200 µL, administered intravenously once per week for four weeks.

