Urolithin A vs Spermidine for Longevity: Benefits, Pathways, and Differences
While urolithin A and spermidine both target cellular maintenance, urolithin A targets the function of the cell’s powerhouses (mitochondria) more, and spermidine has broader pro-longevity effects.
Highlights
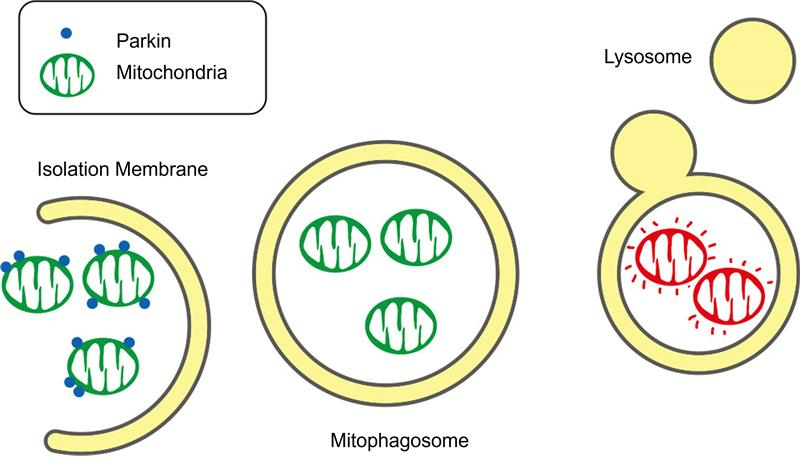
- Research has shown that urolithin A potently stimulates mitophagy—the selective removal and recycling of damaged mitochondria (the cell’s powerhouse).
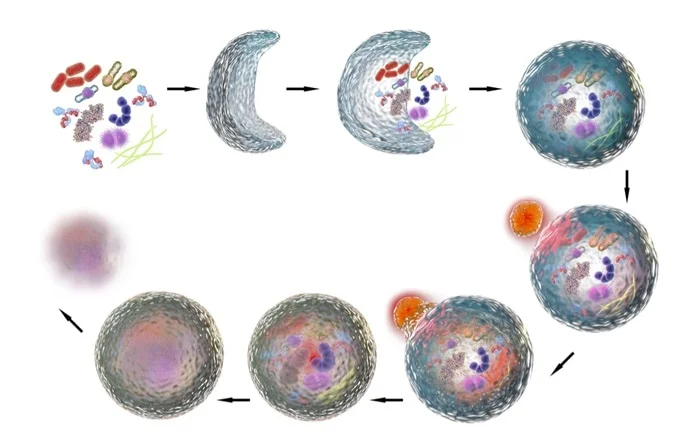
- Research has established that spermidine stimulates autophagy—a cellular mechanism where cells clean out and recycle damaged or dysfunctional components.
- Researchers now contend that spermidine may work better than urolithin A for comprehensive pro-longevity benefits, while urolithin A may work better for muscle endurance, metabolic health, and mitochondrial function.
As published in a review in Nutrition Research Reviews, Borska and colleagues from Charles University in the Czech Republic contend that the supplement urolithin A may work better for individuals seeking to specifically target muscle endurance, metabolic health, and functional capacity of the cell’s powerhouses (mitochondria). However, spermidine may work better than the supplement urolithin A for people seeking comprehensive benefits against aging.
Underlying this contention, the researchers reviewed how both molecules have some degree of influence on two age-related pathways, but urolithin A and spermidine more heavily influence one than the other. Along these lines, urolithin A, to a greater degree, targets mitophagy—the cellular process that selectively removes or recycles damaged mitochondria. Furthermore, spermidine, to a greater degree, targets autophagy—a cellular pathway cells use to clean out and recycle damaged or dysfunctional components. Through their overlapping yet distinct effects on these mechanisms related to cellular and mitochondrial maintenance, urolithin A and spermidine provide different means to address age-related physiological decline. Accordingly, in their review, Borska and colleagues describe details regarding the two supplements’ differing mechanisms of action, preclinical data for their effectiveness, as well as human data.
Urolithin A and Spermidine Have Different Mechanisms of Action
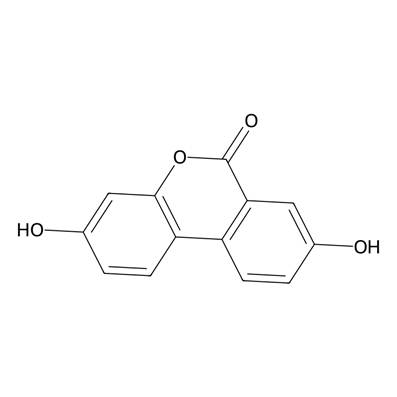
Details on Urolithin A
For some context, urolithin A is a metabolite produced by gut microbes through the transformation of plant-derived compounds, specifically a class of polyphenols known as ellagitannins. These compounds are derived from fruits such as pomegranates, raspberries, strawberries, and walnuts.
Intriguingly, not all people produce urolithin A efficiently, owing to variations in their gut microbe composition. This finding has led to the development of direct supplementation strategies to achieve consistent circulating urolithin A across individuals, regardless of their gut microbial composition.
For supplementation, anywhere between 500 mg and 1,000 mg of oral urolithin A daily has been demonstrated to effectively increase circulating urolithin A. Research has also demonstrated that urolithin A’s bioavailability—the proportion entering circulation after consumption—is highest when consumed in capsule form, compared to consuming ellagitannin-rich fruits. As such, encapsulated urolithin A improves absorption compared to consuming fruits.
Regarding urolithin A’s mechanism of action, the compound induces mitophagy and autophagy; however, research suggests that urolithin A more potently induces mitophagy than autophagy. The cellular mechanism of mitophagy is critical for energy metabolism, particularly in tissues with high energy demands, such as skeletal muscle. Not only does research suggest that urolithin A stimulates mitophagy, but also, to some degree, mitochondrial biogenesis—a crucial cellular process of creating new mitochondria. The combined effects from stimulating mitophagy and mitochondrial biogenesis increase cell energy production and enhance muscle endurance, making urolithin A effective against age-related mitochondrial dysfunction.
In addition to stimulating mitophagy and, to a lesser degree, autophagy, urolithin A exhibits anti-inflammatory and antioxidant properties. Research has demonstrated that urolithin A modulates inflammatory responses by reducing the expression of pro-inflammatory proteins and increasing the production of anti-inflammatory proteins. Moreover, it enhances the activation of antioxidant enzymes like superoxide dismutase, thereby reducing stress from harmful, reactive molecules called reactive oxygen species to protect cells from damage.
The anti-inflammatory and antioxidant properties of urolithin A reduce the risk of age-related chronic inflammatory diseases and support overall cellular longevity. Along these lines, studies in animal models have further demonstrated urolithin A’s efficacy in promoting longevity and improving muscle function. For example, worms administered urolithin A exhibited lifespan extension, and rodents given urolithin A showed enhanced muscle function and reduced signs of muscle aging. Furthermore, human trial research has shown promising results, with older adults exhibiting improved muscle strength and endurance following supplementation. Collectively, these findings highlight urolithin A’s potential to fight age-related muscle decline.
In summary, urolithin A supplementation offers a way to potently target mitochondrial function through the stimulation of mitophagy, which supports muscle function and plays a critical role in cellular health. Also, through its anti-inflammatory and antioxidant properties, urolithin A helps with inflammation control and cellular stress from reactive oxygen species buildup. These attributes position urolithin A as a promising supplement, particularly for older individuals aiming to improve mitochondrial function and mitigate the effects of age-related muscle decline.
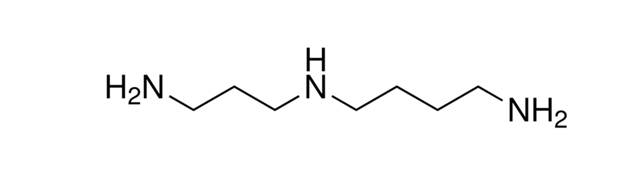
Details on Spermidine
Spermidine is a naturally-occurring polyamine. Polyamines are essential organic compounds found in living cells. Accordingly, spermidine is found in various organisms, including humans. Also, spermidine is found abundantly in plant-based foods, such as wheat germ, soybeans, mushrooms, and aged cheese.
This molecule has garnered attention as a means to support optimal aging, because it plays a crucial role in cellular processes like DNA stabilization and the modulation of cell growth and proliferation, and also because its levels in the body decline with age. As such, some people use it as a supplement to ward off aging, commonly taking orally administered extracts of polyamine-rich plant extract, at doses usually around 750 mg daily.
As for its mechanism of action, spermidine’s potential longevity-promoting benefits arise from its ability to induce autophagy. Through the removal of damaged or unnecessary cellular components, as well as toxins and pathogens, autophagy maintains the balance of cellular function (homeostasis). With its role in maintaining cellular homeostasis, autophagy has been widely implicated in the regulation of aging and longevity.
Spermidine modulates several key signaling pathways, such as AMPK, which regulate energy metabolism, cell stress responses, and cellular longevity. Beyond its influence on autophagy, spermidine affects other cellular processes, like mitophagy. Research has demonstrated that spermidine activates mitophagy, similar to urolithin A, and spermidine’s broader effect on autophagy extends its impact to other cellular components. These effects contribute to spermidine’s protective effect against inflammation and improvement of cellular health, potentially making spermidine a better supplement to target general longevity compared to urolithin A.
The longevity-related benefits of spermidine supplementation extend to various systems of the body, particularly those related to neuroprotective effects. For example, studies with animal models suggest spermidine improves cognitive function and delays neurodegeneration by enhancing autophagy and reducing inflammation in the brain. Moreover, human data provide evidence that spermidine mitigates age-related cognitive decline and improves memory.
Collectively, spermidine is a potent inducer of autophagy, and it stimulates mitophagy to some degree as well. These attributes contribute to spermidine’s broad cellular benefits, ranging from neuroprotection to anti-inflammatory effects. Spermidine’s capabilities to promote cellular homeostasis and cognitive health make it a valuable candidate for supplementation for longevity purposes. Furthermore, spermidine’s ability to modulate key signaling pathways related to cellular longevity, in combination with its availability through supplementation, supports its growing popularity as an aging intervention nutraceutical.
“On the basis of the current evidence, spermidine may be the superior supplement for individuals seeking comprehensive anti-ageing benefits that extend beyond mitochondrial health…” say Borska and colleagues in their publication.
Targeting Autophagy and Mitophagy Simultaneously
An online search does not reveal any preclinical or human studies done to date testing the effects of combining urolithin A with spermidine. Accordingly, future research is necessary to uncover whether any safety issues exist when combining these nutraceuticals, as well as any potential synergistic pro-longevity effects from their combination. In that regard, anyone wanting to take both urolithin A with a spermidine-rich extract needs to be aware of the possibility of as-yet-unknown adverse effects associated with their combination.
Interestingly, Seragon’s Restorin contains proprietary autophagy and mitophagy activators, so using Seragon’s product may serve as another way to simultaneously promote autophagy and mitophagy. Along these lines, preclinical data show that SRN-901, a drug Restorin is roughly based on, dramatically extends remaining lifespan in aged rodents. This finding offers evidence that the product indeed has beneficial effects on age-related cellular health pathways.