Emerging Therapy Reverses Memory Loss and Boosts Brain Connectivity in Aged Monkeys
Scientists ameliorate age-related deficits in memory and brain structure by administering vesicles secreted from stem cells to monkeys.
Highlights
- Stem cell vesicles reverse age-related memory impairments in monkeys.
- The improvements in memory are associated with increased brain connectivity.
- We reiterate the role of NMN in the efficacy of stem cell vesicle therapy.
Picture a dog and a rabbit. Now imagine them racing each other. Easy enough? Now add a cat to the race. A bit more difficult? Now, without reading backwards, add a turtle and a horse, and visualize all five animals racing each other. In this case, you may have lost track of all the animals. Why? Because psychological research suggests we can only hold and manipulate three to five items in our minds at a time.
This cognitive ability, to hold and manipulate information, is called working memory, and it declines with age. Our working memory is the human equivalent of a computer’s random-access memory (RAM). As declining RAM slows down a computer’s performance, declining working memory slows down our cognitive performance. However, scientists from Boston University may have found how to reverse working memory loss.
As published in a new study in Geroscience, Dr. Tara Moore and her team ameliorated age-related working memory deficits in rhesus monkeys. Moreover, Moore and team showed that the improvements in working memory correlated with enhanced brain connectivity. How? With extracellular vesicles.
Extracellular Vesicles (EVs)
EVs are vesicles secreted by cells into the extracellular space—the space surrounding cells. They act as molecular couriers, delivering cargo to cells throughout the body. Each type of cell secretes EVs with a distinct set of molecules, reflecting the properties of that cell. For example, stem cells, which are capable of regenerating tissues, secrete EVs that carry molecules capable of regenerating tissues.
While discovered in the 1940s, EVs were initially thought to be “garbage bins” for cellular waste. It wasn’t until the late 1990s that scientists began to realize their profound physiological effects. More recently, a 2024 study catalogued 471 EV-related clinical trials investigating their effects on over 200 diseases, suggesting the emergence of EVs as a viable therapy. However, less is known about their effects on age-related conditions like cognitive decline.
Regenerating the Degenerating Brain
While the initial molecular triggers of cognitive decline are a subject of active research, at the macroscopic level, it is characterized by brain degeneration. This is especially true for more severe forms of cognitive impairment, which manifest with increasingly higher probability as we age. Brain degeneration may not necessarily be due to neuron loss, but encompasses the deterioration of myelin, the layer of fat that coats our nerve fibers.
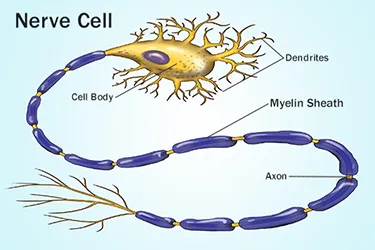
Myelin acts as an insulator, allowing electrical signals to travel at speeds over 10 times faster than without myelin. In that respect, myelin is crucial for speeding up connectivity between different brain regions. The “disconnection hypothesis” states that disruptions in brain connectivity may explain age-related cognitive decline. The hypothesis is supported by a study showing that the degradation of myelin is associated with cognitive decline in aging adults.
The idea behind stem cell EVs (EVs secreted by stem cells) is that they can regenerate tissues that have degenerated. This includes tissues, such as myelin (a specialized fatty tissue), that have degenerated due to aging. The hope is that, in the future, stem cell EVs can be administered therapeutically to combat cognitive decline. By doing so, the contents of the EVs may be delivered to the brain to regenerate lost tissue, leading to improvements in cognitive performance.
Stem Cell EVs Improve Working Memory
To begin the study, thirteen monkeys, equivalent in age to 51- to 72-year-old humans, received a working memory test to obtain baseline measurements. As expected, the monkeys exhibited no differences in working memory. Subsequently, about half the monkeys were injected with EVs for 18 months. Meanwhile, the other half did not receive EVs.
Remarkably, after 18 months, the monkeys receiving EV treatment showed improvements in working memory. In stark contrast, the monkeys not receiving EV treatment saw a decline in working memory. These findings suggest that stem cell EV therapy not only prevents working memory decline, but the EVs may reverse impairments in this valuable cognitive faculty.
“These striking results indicate that [stem cell EV] treatment may not only slow age-related declines in working memory but also reverse age-related impairment and improve memory performance in this cohort of middle-aged monkeys,” said the authors.

Stem Cell EVs Improve Brain Connectivity
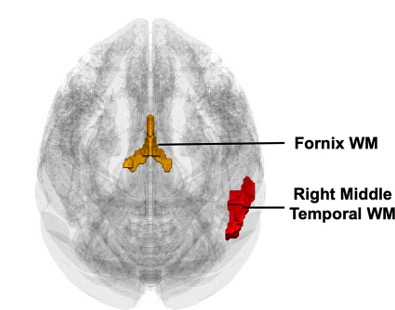
To assess brain connectivity, the monkeys were scanned with an MRI machine. Brain MRI scans consist of darker regions called gray matter and lighter regions called white matter. Gray matter represents the cell bodies of neurons (somas) while white matter represents myelinated nerve fibers (myelinated axons). Thus, Moore and team examined the monkey’s white matter to estimate their nerve fiber integrity and myelination.
They found that stem cell EV treatment increased white matter in two key brain regions:
- The fornix: A major white matter tract involved in learning, which projects from the hippocampus, a brain region essential for new memory formation.
- The right middle temporal region: A brain region that plays a crucial role in sensory integration and the formation of new concepts.
The authors point out that the right middle temporal region is not traditionally emphasized in working memory. However, it serves as an integrative region that supports memory processing. Importantly, the increased white matter in these regions was correlated with improvements in working memory. It follows that these improvements in brain connectivity may potentially be responsible for improving the working memory of aged monkeys.
Overall, the study suggests that stem cell EVs regenerate the myelin of nerve fibers to improve neuronal health and conduction speed, leading to improvements in working memory. The authors of the study conclude,
“This study highlights the potential of [stem cell EVs] as a viable, minimally invasive therapeutic approach for promoting healthy cognitive aging in higher-order primates, with implications for translational development in aging human populations.”
Challenges
EVs are considered a cell-free therapy, offering major advantages over cell-based therapies like stem cell therapy. Examples include, lower risk of tumor growth and a lower likelihood of immune rejection. EVs are also easier to produce, store, and transport than stem cells. However, before EV therapy can be widely used on humans, several challenges must be addressed, primarily related to the safety, quality control, and regulatory oversight of large-scale manufacturing.
Combating Aging and the Role of NMN
We have previously reported on studies showing that EVs can counteract heart aging and cellular senescence. Additionally, NMN may enhance the efficacy of EVs, especially when it comes to preventing heart attack-induced cardiac tissue degeneration. With that said, it may be possible that stem cell EV therapy can ameliorate nearly all aspects of degenerative aging. This is because most age-related diseases are a result of organ and tissue degeneration. However, there is still a lot more to learn before this may become a reality.

