How Innovative Therapies Are Tackling the Root Causes of Aging
Aging encompasses a gradual decline in cellular and physiological function, and some potential strategies, such as NAD+ precursors and rapamycin, may help address this decline.
Highlights
- Strategies such as NAD+ precursors target primary hallmarks of aging—considered the root causes of aging.
- Therapeutics like rapamycin target antagonistic hallmarks of aging—compensatory characteristics in response to root causes of aging that become detrimental over time.
- Compounds like senolytics target integrative hallmarks of aging—features that reflect systemic decline.
Aging is not merely the number of years a person has lived (referred to as chronological age) but can also be gauged by the actual condition of cells, tissues, and organs (a concept known as biological age). Accordingly, assessing biological age can be thought of as a way to reveal how quickly the body is aging.
Along those lines, biological age is modulated by the body’s ability to maintain a stable and functional internal environment in the face of internal and external stressors, an idea known as maintenance of dynamic equilibrium. Maintaining dynamic equilibrium supports crucial physiological processes, like cellular repair, metabolic regulation, and immune function. When dynamic equilibrium is disrupted during aging, it can lead to physical decline, which in turn accelerates aging and increases susceptibility to age-related diseases.
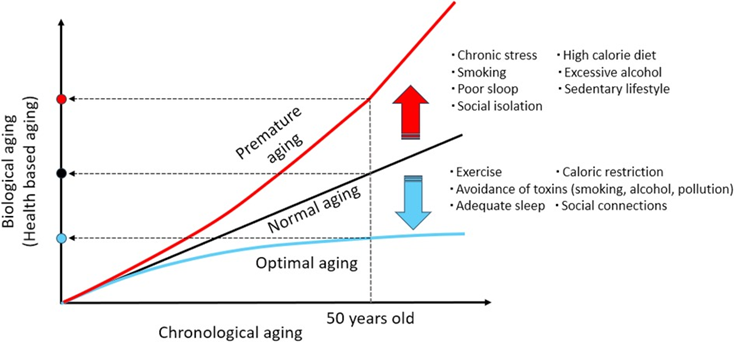
The hallmarks of aging, physiological features that serve as key drivers of biological aging, determine, at least partially, whether dynamic equilibrium is maintained. For this reason, in 2013, researchers consolidated the hallmarks of aging framework based on scientific insights on the mechanisms of aging in an attempt to identify potential physiological routes of intervention against aging. Then, in 2023, researchers updated the hallmarks of aging to incorporate a further decade’s advances in aging research.
Accordingly, each hallmark of aging entails a specific alteration to molecular or cellular characteristics, which can often be measured with established physiological markers of aging, such as proteins assessed during blood work. Moreover, these hallmarks of aging progress during aging processes over time in distinct, yet interrelated stages, which collectively contribute to aging and age-associated diseases.
Ongoing research seeks to unravel the optimal time points during life for therapeutic intervention and to identify lifestyle factors that could delay aging or prevent premature aging. Accordingly, as published in Frontiers in Cardiovascular Medicine, Morishita and colleagues from Osaka University in Japan delve into the current state of knowledge and related findings from preclinical research on the hallmarks of aging. Their insights may help anyone interested in deciding which aging interventions to use.
Three Groups of Hallmarks and Possible Interventions Against Them
The hallmarks of aging can be seen as critical biological processes driving the decline in physiological function and increasing risks for age-related diseases as people get older. However, with recent aging research advances, targeting fundamental molecular and cellular processes related to the hallmarks of aging can potentially modulate biological aging.
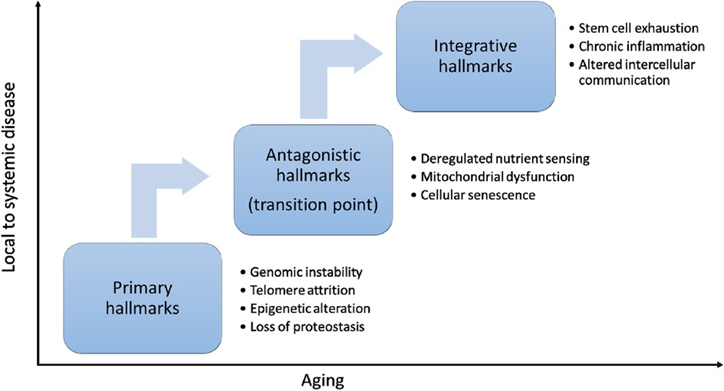
Targeting the Primary Hallmarks of Aging
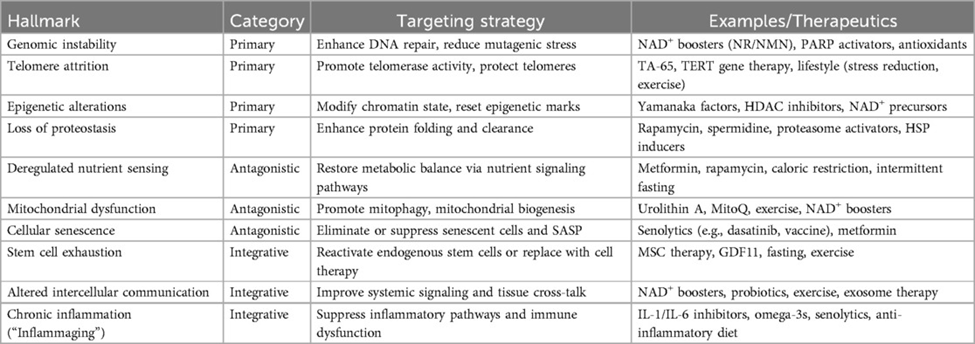
The primary hallmarks of aging are considered the root causes of cellular aging. They include things like chromosomal (genomic) instability; shortening of the protective ends of chromosomes, composed of repetitive DNA sequences (telomere shortening); alterations to molecular tagging patterns on DNA (epigenetic alterations); and the loss of stable, functioning proteins (loss of proteostasis). These hallmarks are associated with a variety of age-related diseases: DNA damage accumulation is associated with cancer, telomere shortening contributes to a condition characterized by lung scarring (idiopathic pulmonary fibrosis), epigenetic alterations underlie neurological conditions like Alzheimer’s disease, and loss of proteostasis is linked to other neurodegenerative conditions like Parkinson’s disease.
Interventions against these hallmarks of aging include gene therapy with the enzyme telomerase to counteract telomere shortening and nicotinamide adenine dinucleotide (NAD+) precursors like nicotinamide mononucleotide (NMN) to support DNA repair and genomic stability. To counteract the accumulation of epigenetic alterations with age, Harvard’s Dr. David Sinclair and his team have developed a method called partial cellular reprogramming, which involves treating cells with proteins known as Yamanaka factors. For the restoration of proteostasis, compounds that enhance autophagy (the cell’s means to eliminate and recycle dysfunctional proteins), such as rapamycin, have shown promise in preclinical models.
Drawbacks to translating the promising effects of these interventions in preclinical models to humans include cancer risks and insufficient data on the long-term safety of usage. For example, therapies that stimulate cellular activity, such as telomerase gene therapy, may paradoxically accelerate aging if not precisely regulated, leading to an aging-related cellular state of dysfunction known as senescence, as well as the proliferation of cancer cells. Thus, researchers need to uncover ways of precisely targeting tissues with these therapies, as well as combinations of these therapies, to achieve physiological rejuvenation without compromising long-term safety.
Targeting the Antagonistic Hallmarks of Aging
The antagonistic hallmarks of aging initially serve as protective and adaptive responses to the primary hallmarks but become damaging over time. These antagonistic hallmarks include physiological features like dysfunction of the cell’s powerhouse (the mitochondria) and cellular senescence.
Interestingly, mitochondrial dysfunction, characterized by impaired metabolism and the buildup of harmful, reactive molecules called reactive oxygen species in cells, plays a key role in age-related diseases like Alzheimer’s and Parkinson’s as well as heart conditions. Moreover, cellular senescence, characterized by the secretion of inflammatory factors collectively called the SASP, contributes to age-related diseases like osteoporosis, osteoarthritis, lung fibrosis, and cancer by promoting inflammation and tissue dysfunction.
Modulating these antagonistic hallmarks of aging is a key goal in aging research, with strategies including the use of compounds called senolytics that selectively eliminate senescent cells, and compounds that mimic dietary calorie restriction, like rapamycin. While preclinical research shows promise for treatments like senolytics and rapamycin extending average lifespan, finding whether the pro-longevity benefits of these therapies apply to humans has remained a challenge. For that reason, more clinical research is necessary to disentangle whether senolytics and compounds mimicking calorie restriction alleviate signs of aging in people.
Targeting Integrative Hallmarks of Aging
With the age-related accrual of cellular damage and the failure of cellular mechanisms aimed at compensating for damage, integrative hallmarks of aging emerge. Such integrative hallmarks of aging include physiological characteristics like stem cell exhaustion (a decline in the number and function of stem cells), inflammation, and altered communication between cells. These physiological features of aging reflect a systemic functional decline driven by hallmarks of aging that arise earlier during the aging process, from the primary and antagonistic hallmarks of aging.
Furthermore, the integrative hallmarks of aging contribute to age-related conditions like sarcopenia (the age-related loss of skeletal muscle and mass) and immune dysfunction (where stem cell depletion hinders immune cell regeneration). Other age-related conditions believed to arise from the integrative hallmarks of aging are chronic inflammatory diseases like atherosclerosis, Alzheimer’s disease, and diabetes, all exacerbated by systemic inflammation.
Therapeutic options targeting the integrative hallmarks of aging include stem cell transplantation (a medical procedure that replaces damaged stem cells with healthy ones), plasma exchange (a procedure that extracts blood and replaces the plasma fraction with a replacement fluid), and senolytics like dasatinib and quercetin. Potential pitfalls of these approaches include short-lived effects. Thus, more clinical research is necessary to unravel how long the effects of these therapies may last to confirm their efficacy against the integrative hallmarks of aging.
Identifying Products Targeting Multiple Hallmarks of Aging
Interestingly, some products already on the market target multiple hallmarks of aging. Accordingly, Seragon’s Restorin has proprietary senolytic, autophagy-activating, mitophagy-activating (where dysfunctional mitochondria are degraded), anti-inflammatory, and NAD+ precursor technologies.
With senolytic components, Restorin targets the antagonistic hallmarks of aging, including cellular senescence, and the integrative hallmarks of aging, including chronic inflammation. Moreover, autophagy activators contained in Restorin target primary hallmarks of aging, like loss of proper protein function, by enhancing dysfunctional protein clearance. Furthermore, mitophagy activators in Restorin target antagonistic hallmarks of aging, like mitochondrial dysfunction, by degrading dysfunctional mitochondria. The anti-inflammatory technologies in Restorin also target an integrative hallmark of aging, chronic inflammation. Finally, NAD+ precursors contained in Restorin target the primary hallmarks of aging, genomic instability and epigenetic alterations, an antagonistic hallmark of aging, mitochondrial dysfunction, and an integrative hallmark of aging, altered communication between cells.
In this way, Restorin targets an array of hallmarks of aging, spanning the three groups of hallmarks—primary, antagonistic, and integrative hallmarks. Hence, using a product like Restorin may serve as a way to target a wide swath of the hallmarks of aging.

