Hydrogel Laced With Immune Cell Secretion Enhances Muscle Regeneration
Immune cell signals that activate muscle stem cells like NAMPT have therapeutic potential for muscle injury and disease
Highlights
- Immune cells called macrophages are required for muscle repair.
- These macrophages stimulate muscle stem cell proliferation by secreting an NMN generating protein called NAMPT.
- Injured muscles in fish and mice can be repaired and regenerated with NAMPT treatment.
No matter our age, our muscles are always undergoing wear and tear. To keep our muscles fresh and our bodies mobile, we use a system with stem cells that live in our muscles that help repair them. But as we get older, these muscle stem cells lose their ability to regenerate our muscles. Triggering these stem cells is at the crux of fixing muscle damage, whether it’s from daily use, an injury, disease, or age.
Ratnayake and colleagues from Monash University in Melbourne, Australia, published an article in Nature revealing a factor that triggers the proliferation of these stem cells to heal muscles. By studying the cells that migrated to a muscle injury in zebrafish, the researchers identified a group of immune cells, called macrophages, that stimulated stem cells to regenerate by secreting a protein called nicotinamide phosphoribosyltransferase (NAMPT) at the site of muscle injury. Injecting this naturally occurring protein within a hydrogel completely regenerated injured muscle and restored its capability for movement after severe muscle trauma.
“This study demonstrates that macrophage-derived niche signals for muscle stem cells, such as NAMPT, can be applied as new therapeutic modalities for skeletal muscle injury and disease,” concluded the authors. “Providing specific macrophage-derived signals required for muscle stem cells proliferation, such as the one we identified here, will provide an avenue to achieve better [muscle stem cell]-based therapy outcomes.”
What’s the missing link in muscle regeneration?
The muscle stem cell is the posterchild of a unipotent tissue-resident stem cell that occupies a specific anatomical niche within matured tissue. Decades of research have revealed the extraordinary capacity of this system to effectively coordinate muscle repair in response to a wide variety of insults and injuries. Despite this, transplantation of isolated muscle stem cells has yet to provide therapeutic effects, and pro-regenerative treatments that stimulate muscle stem cells are lacking at this juncture.
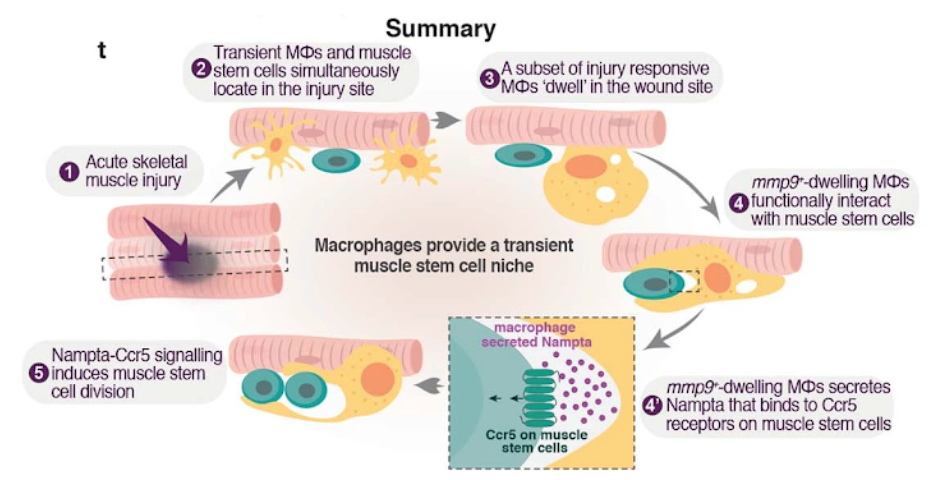
Macrophages secrete NAMPT to stimulate muscle stem cells
Ratnayake and colleagues found that a specific subset of a type of immune cell called macrophages that ‘dwell’ within muscle injuries in zebrafish rapidly insulated lesions in muscle fibers after an injury. This specific macrophage subset then appeared to control muscle regeneration by creating a pro-proliferative niche for the muscle stem cells. “What we saw were macrophages literally cuddling the muscle stem cells, which then started to divide and proliferate. Once they started this process, the macrophages would move on and cuddle the next muscle stem cell, and pretty soon the wound would heal,” said Peter Currie, director of Monash University’s Australian Regenerative Medicine Institute in Melbourne.
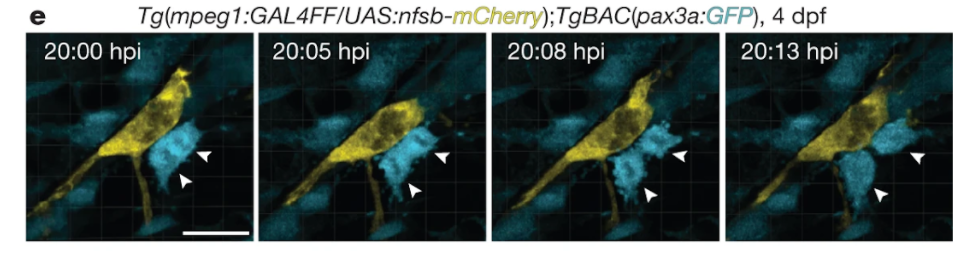
The Monash University-led research team then pinpointed proliferative signals secreted by these muscle-cuddling macrophages in zebrafish. This included the secreted protein NAMPT, an enzyme critical to NAD+ metabolism that generates nicotinamide mononucleotide (NMN). They found that NAMPT was acting through a receptor called CCR, which is found on muscle stem cells. When they removed Nampt genetically from these macrophages so that they could not secrete it, the muscle regeneration response faltered. But, when they added a human version of NAMPT into the zebrafish tanks, the muscle regeneration response was restored. This shows that the macrophage niche, by secreting NAMPT, is required for muscle regeneration in zebrafish,
NAMPT promotes muscle regeneration in mice
Ratnayake and colleagues then went on to investigate whether externally delivered NAMPT could facilitate regeneration in mice that underwent a severe muscle injury. Notably, when they delivered human NAMPT into the muscle defect using a fibrin hydrogel but not a fibrin-only control hydrogel, the muscle architecture was fully restored when applied to the wound site. These findings reveal that supplemented NAMPT stimulates muscle repair in the context of an acute injury of adult mammalian muscle in a similar manner to the results described for zebrafish.
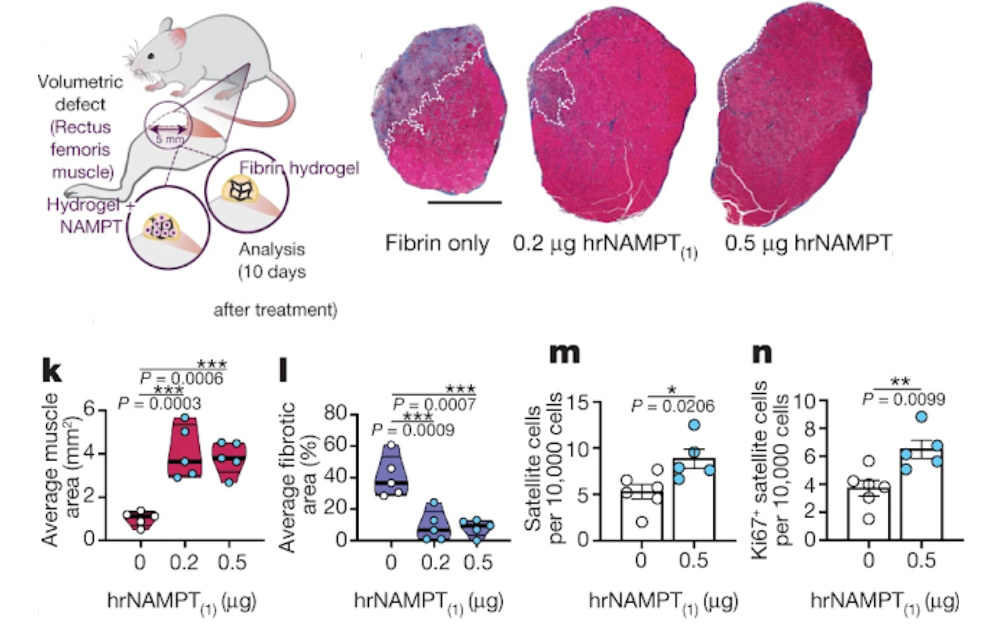
“This analysis shows that in addition to their ability to modulate the immune response, specific macrophage populations also provide a transient stem-cell-activating niche, directly supplying proliferation-inducing cues that govern the repair process that is mediated by muscle stem cells,” said the authors. “This work may also contribute to more knowledge about the role of macrophages in the heart,” proposed Jeroen Bakkers, group leader at the Hubrecht Institute.