Dr. Peter Attia and Longevity Expert Brian Kennedy Reveal Aging Secrets
Biologist Brian Kennedy joins Peter Attia to discuss rapamycin trials, aging models, biological clocks, and lifestyle tools that may extend human healthspan.
Highlights:
- Brian Kennedy outlined two models of aging: one linear and one exponential, arguing that understanding both is essential for designing interventions that preserve resilience and extend healthspan.
- Kennedy and Attia discussed the limitations of current aging biomarkers and how Kennedy’s team is developing more actionable biological clocks to measure the impact of longevity interventions.
- Kennedy shared insights from ongoing human rapamycin trials, highlighting how dose, timing, and exercise may shape its effectiveness in slowing biological aging.
Aging has long been seen as an unavoidable biological decline. However, a recent episode of The Drive podcast, hosted by Peter Attia, featured a conversation with longevity scientist Dr. Brian Kennedy that explored whether the biology of aging can be measured, influenced, or even delayed through emerging interventions.
Kennedy, a researcher with decades of experience in the aging field and a leader of translational efforts in Singapore, discussed the current state of longevity science and what tools might actually deliver meaningful extensions in human healthspan. The interview covered experimental compounds, new biological models of aging, and how researchers are shifting from disease treatment to prevention through resilience-based strategies.
The Central Role of Resilience in Aging
Kennedy describes aging as a loss of physiological resilience. In youth, the body can return to equilibrium after stress, injury, or illness, maintaining healthy function across systems. Over time, that homeostatic ability diminishes. Minor challenges begin to have disproportionate consequences, leading to chronic disease, frailty, and reduced quality of life.
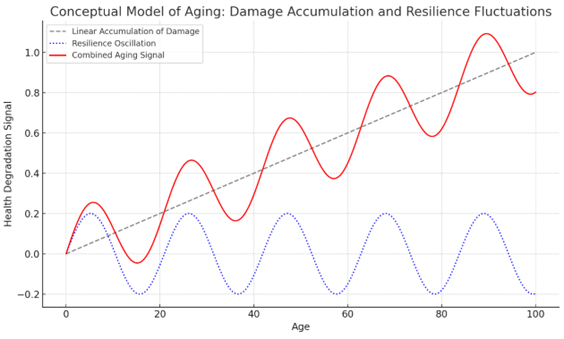
This concept is being studied through mathematical models using large human datasets such as the UK Biobank. These models suggest that aging involves two overlapping patterns: a linear accumulation of damage or dysregulation, and an oscillating function that represents day-to-day or month-to-month fluctuations in health. Many current interventions appear to impact the second pattern, improving how well someone functions relative to their age, but do not alter the long-term trajectory of decline.
Aging Clocks and the Measurement of Biological Time
Biological aging clocks have become a central tool in longevity research. These clocks aim to estimate an individual’s biological age using molecular markers such as DNA methylation patterns, gene expression profiles, proteomic data, and immune system activity. Clocks are being used in clinical studies to measure whether interventions produce measurable changes over time.
Kennedy noted that while methylation-based clocks receive the most attention, other types, including proteomic, metabolomic, and even facial structure-based clocks, have shown comparable accuracy. Many of these tools are still experimental, and few have been validated for clinical use. However, they are already becoming useful in early-stage trials, where measuring aging directly remains a challenge.

Rapamycin Trials in Humans
Rapamycin, an mTOR inhibitor originally developed as an immunosuppressant for organ transplant patients, remains one of the most studied and promising drugs in the aging field. In multiple animal models, rapamycin has extended lifespan, improved immune function, and reduced age-related pathology.
Kennedy described an ongoing clinical trial his team is conducting in Singapore. In this study, healthy adults aged 40 to 60 are receiving a low dose of rapamycin (5 milligrams once weekly) for six months. The trial is measuring a broad range of outcomes, including blood-based inflammatory markers, biological aging clocks, arterial stiffness, cognitive performance, and muscle strength.
The goal of the trial is not to treat a disease but to determine whether rapamycin can improve markers of health and resilience in people without diagnosed conditions. This approach reflects a shift in longevity research toward preventive strategies aimed at delaying or mitigating the onset of multiple chronic diseases simultaneously.
Promising Natural Compounds: AKG, Urolithin A, and Spermidine
Alongside pharmaceutical agents, Kennedy discussed several natural compounds that are being investigated for their potential to support healthy aging. These include alpha-ketoglutarate (AKG), urolithin A, and spermidine.
AKG, a central metabolite in the Krebs cycle, has shown the ability to extend lifespan and dramatically reduce frailty in mouse models. A time-release version of AKG is currently being tested in humans to assess its effects on methylation clocks, inflammation, and functional health measures. While results from this trial have not yet been published, Kennedy indicated that preliminary data are encouraging.
Urolithin A is a compound produced by gut bacteria from dietary polyphenols found in foods like pomegranates. It is believed to enhance mitochondrial quality by promoting mitophagy, the process by which cells remove damaged mitochondria. In preclinical studies, urolithin A improved muscle function and reduced frailty in aging animals. Kennedy noted that while data in humans remains limited, early evidence supports its continued investigation.
Spermidine, a naturally occurring polyamine found in certain fermented foods, is another candidate being evaluated. It is known to induce autophagy and has been associated with lifespan extension in model organisms. Together, these compounds, while distinct in their mechanisms, share a common feature: they appear to support cellular maintenance pathways that are active during caloric restriction and other stress-adaptive states.
Research Gaps and the Limits of Translating Animal Studies to Humans
Despite growing interest in these interventions, Kennedy emphasized that the science remains early. Many studies in animals may not translate directly to humans, and the effects observed in simpler organisms like worms or yeast can be misleading due to fundamental biological differences.
Additionally, regulatory constraints prevent drugs from being approved solely for the purpose of slowing aging. As a result, many longevity trials must use surrogate endpoints such as epigenetic age, inflammatory markers, or physical performance measures.
Commercial interest in longevity has also led to a proliferation of products marketed with minimal evidence. Kennedy cautioned against overinterpreting early findings and called for more funding for academic studies that explore the basic mechanisms of aging. Unlike commercial ventures, these studies are better positioned to ask foundational questions about the biology of aging and resilience.
Why Targeting the “Hallmarks of Aging” May Miss the Bigger Picture
The discussion also addressed the widely cited “hallmarks of aging” framework, which includes factors such as mitochondrial dysfunction, genomic instability, and cellular senescence. While these hallmarks have helped organize the field, Kennedy warned against treating them as isolated targets.
In practice, interventions that appear to slow aging, such as rapamycin or exercise, tend to affect many hallmarks at once. This suggests that aging is better understood as a networked process involving feedback loops between inflammation, nutrient signaling, stress response, and damage repair.
The most effective strategies may not be those that target a single node in this network, but those that restore the body’s ability to maintain equilibrium in the face of stress and injury.
Longevity Goals in Perspective: Healthspan Gains Over Immortality
While the idea of radically extending human lifespan often captures public attention, Kennedy offered a more measured perspective. He described most current interventions as capable of adding five to ten years of high-quality life for many people, especially when combined with healthy behaviors such as exercise, sleep, and nutrition.
Achieving the kind of maximal lifespan increases imagined in science fiction would likely require a new class of interventions, possibly involving cellular reprogramming, genetic modification, or more precise control of biological systems. These approaches remain speculative.
For now, the focus remains on improving healthspan, the number of years lived without chronic disease or disability. As Kennedy put it during the podcast, expanding the healthy phase of life would represent a major breakthrough in medicine, even if it does not lead to extreme longevity.

