Supplementing NAD+ Preserves Neurons and Memory Formation in Rats with Hypoglycemia
Research indicates nicotinamide mononucleotide (NMN) supplementation may prevent cognitive impairment in diabetes patients.
Hypoglycemia, where blood glucose drops below normal, often comes from insulin treatment for diabetes. It can also cause brain injury and in severe cases drives neuronal death and cognitive impairment. Trying to control blood glucose with insulin reduces the risk of diabetes complications but that of severe hypoglycemia increases. Currently, there is no known therapeutic option for attenuating hypoglycemia-induced neuronal death and the resulting cognitive deficits.
Recently, Sakurai and colleagues from the National Center for Geriatrics and Gerontology in Japan and the First Affiliated Hospital of China Medical University published a study in the Brain Research Bulletin that suggested that the molecule nicotinamide mononucleotide (NMN) may hold promise as a therapeutic option to prevent brain injury from hypoglycemia. In the study, they used a rat model with insulin-induced severe hypoglycemia and injected them with NMN 30 minutes after severe hypoglycemia. NMN injections reduced neuronal damage, enhanced cognitive abilities, and improved long-term potentiation—a cellular indicator of memory formation. “These results suggest that NMN may be a promising therapeutic drug to prevent hypoglycemia-induced brain injury,” said the team in their publication.
Restoring NAD+ Levels to Attenuate the Effects of Hypoglycemia on Brain Function
NMN is a precursor to the molecule nicotinamide adenine dinucleotide (NAD+), and supplementation with NMN can increase cellular levels of NAD+ in aged rodents and possibly in people. Cells need NAD+ for energy production and maintenance of DNA integrity, and scientists have linked reduced NAD+ levels with age to diseases like Alzheimer’s disease, metabolic disorders, and cardiovascular complications.
Restoring NAD+ levels with NMN supplementation may ameliorate cellular imbalances caused by hypoglycemia. In hypoglycemia, harmful molecules called reactive oxygen species accumulate in neurons, causing DNA damage and activation of proteins called PARPs that repair the DNA and which also consume NAD+ to perform their function. Activation of PARPs results in depletion of NAD+ and neuronal death since neurons require healthy levels of NAD+ for cellular energy production and metabolic function.
NMN Injections Preserve Neurons and Cognition Following Hypoglycemia
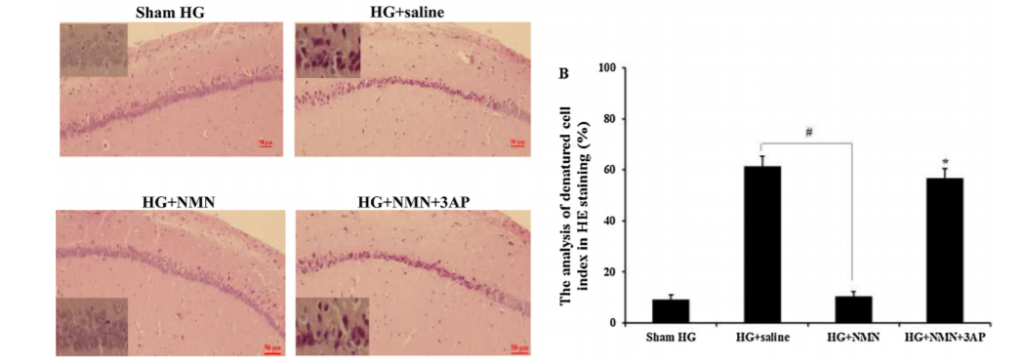
Sakurai and colleagues found that NMN reduced neuronal death after insulin-induced severe hypoglycemia. Neurons are normally round and clear with intact cellular structures, but following hypoglycemia, the team observed degeneration and death of neurons along with a loss of neurons in the brain. These changes were substantially abated in rats that received injections of glucose plus NMN (500 mg/kg) after hypoglycemia. The addition of the chemical 3AP, which renders NAD+ inactive with NMN, abolished NMN’s preservation of neurons, indicating that NMN’s effects came from boosting NAD+ levels.
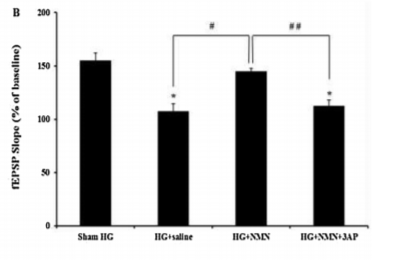
Administering NMN prevented severe hypoglycemia-induced inhibition of a cellular process indicative of memory formation called long-term potentiation. The scientists measured long-term potentiation by performing electrical recordings from neurons one week after hypoglycemia. They found increased electrical activity in normal neurons 50 minutes after an electrical stimulation training period, but this measurement of long-term potentiation was blunted after hypoglycemia. NMN restored long-term potentiation following hypoglycemia as measured with electrical recordings.
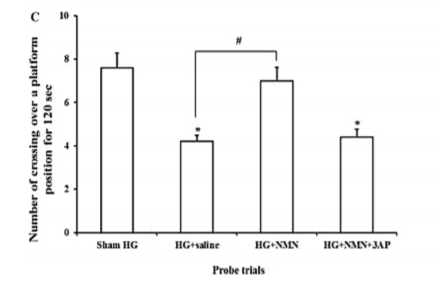
When examining the rats’ behavior, the investigators observed that NMN administration prevented cognitive deficits after severe hypoglycemia. Hypoglycemia impeded the rats’ performance in a spatial memory test called the water maze. When rats were supplemented with NMN, their performance improved in the water maze.
“We investigated the effects of NMN on severe hypoglycemia-induced brain injury. These results showed that NMN administered reduced the neuronal cell death and cognitive impairment that resulted from severe hypoglycemia,” stated the scientists in their publication.
Hypoglycemia can lead cells to make harmful molecules called reactive oxygen species. These molecules cause DNA damage and activate NAD+-dependent proteins called PARPs that repair damaged DNA. However, PARP activation causes NAD+ depletion, which can inhibit metabolism in neurons and lead to neuronal death. NMN increased NAD+ levels in rats, which attenuated neuronal death and improved cognition following hypoglycemia.
“NMN may protect neurons against hypoglycemia-related cognitive impairment by elevating antioxidant defenses,” said the investigators in the article.
Can NMN Preserve Cognitive Function in Diabetes Patients with Severe Hypoglycemia?
Sakurai and colleagues say NMN treatment may be an effective intervention in diabetes patients with severe hypoglycemia. Researchers will need to perform clinical trials to determine whether NMN holds these benefits for diabetes patients.

