Scientists Demonstrate Increasing NAD+ Levels Restores Mitochondrial Function and Limits Aging
A recent study presents data showing nicotinamide adenine dinucleotide (NAD+) restoration limits mitochondrial dysfunction and aging in Werner syndrome.
A study led by Hilde Nilsen and Vilhelm A. Bohr of University of Oslo, Norway and University of Copenhagen, Denmark, respectively, along with scientists from the National Institutes of Health in Baltimore, Maryland, USA, presents data showing nicotinamide adenine dinucleotide (NAD+) restoration limits mitochondrial dysfunction and aging in Werner syndrome.
Studies indicate a cellular process called autophagy, which entails cleaning debris and dysfunctional cellular components (organelles) from the cell plays a role in aging. In order to maintain a steady state balance (equilibrium) for health, cells frequently dispose of and recycle organelles through this process. During autophagy, cell structures (autophagosomes) take up and target small proteins and organelles to the ‘waste bin’ of the cell (the lysosome). In fact, degradation and recycling of organelles (the process of autophagy) occurs less frequently with aging.2 Moreover, longevity-promoting regimens like caloric restriction associate with improved autophagy.3
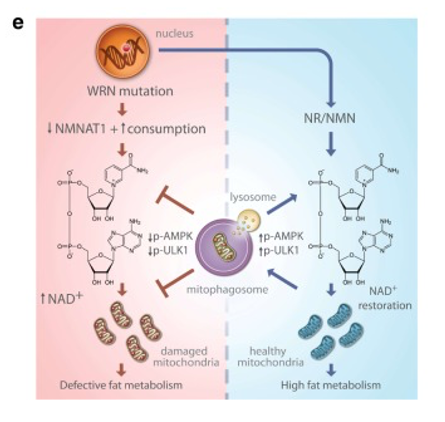
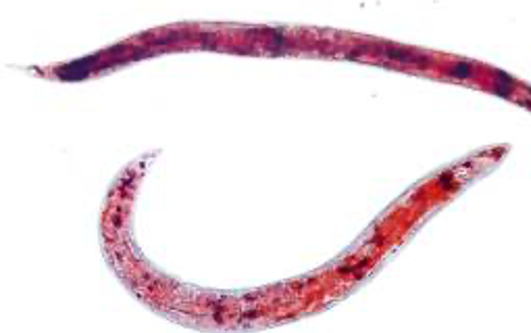
Mitophagy constitutes a type of autophagy whereby mitochondria in cells get degraded and recycled. Compromised mitophagy processes may cause mitochondrial dysfunction increasing with age.2 The scientists propose in the study Werner syndrome, a disease causing accelerated aging, has a disease process (etiology) involving “dysregulation of mitochondrial homeostasis.1” In other words, the group believes function of mitochondrial processes has dysfunctional properties in Werner syndrome. The group proposes (hypothesizes) mitophagy diminishes with age to facilitate dysfunctional mitochondrial function. In order to test this, the group uses roundworms, C. elegans, as models of Werner syndrome. The group includes cells (fibroblasts) from patients with Werner syndrome for testing as well.
The scientists find unhealthy characteristics, including damaged mitochondria, in cells from Werner syndrome patients compared to cells from healthy individuals. In the first part of the study, the team wants to compare mitochondrial dysfunction in Werner syndrome to mitochondria from healthy cells. The team uses cells (fibroblasts) from a patient with Werner syndrome to compare with cells (fibroblasts) from a healthy person. The mitochondria of fibroblasts from the patient with Werner syndrome have unhealthy characteristics in comparison to mitochondria from fibroblasts of a healthy individual. Measurements of unhealthy characteristics of mitochondria include lower mitochondrial membrane potential (indicating greater permeability), higher content of mitochondria, and decreased energy molecules (ATP) in Werner syndrome fibroblasts compared to fibroblasts from a healthy individual. Using electron microscopy, the scientists find fibroblast cells from the Werner syndrome patient have damaged mitochondria compared to fibroblast cells from a healthy person. The group then runs similar experiments in the roundworm model, C. elegans, and finds similar results regarding mitochondria in Werner syndrome model worms compared to healthy worms.
The group finds plasma levels of NAD+ are, on average, 58% lower in the Werner syndrome patients compared to healthy individuals. The scientists measure NAD+ levels in Werner syndrome fibroblasts compared to fibroblasts from a healthy individual. Similar results regarding NAD+ levels are obtained from Werner syndrome model roundworms compared to healthy roundworms.
Treating Prematurely Aged Worms with NAD+ Precursors Extends Life
The lifespan of Werner syndrome model roundworms treated with nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN) improves to nearly the lifespan of healthy roundworms. The scientists start with asking whether replenishing NAD+ will limit accelerated aging in animal models of Werner syndrome. Roundworms with Werner syndrome (C. elegans Werner syndrome model) and healthy roundworms are treated with NR, the NAD+ precursor. The group also treats roundworm Werner syndrome model worms with NAD+ precursor, NMN. Similar aging benefits result with NMN treatment as with NR.
Treatment with NR and NMN, NAD+ supplements, increases NAD+ levels and improves mitophagy to normal levels in Werner syndrome worms. The scientists think impaired mitophagy resulting from decreased NAD+ levels in Werner syndrome contributes to reduced mitophagy. They believe reduced mitophagy facilitates presence of damaged mitochondria in Werner syndrome since mitophagy gets rid of damaged mitochondria. In order to test this, the group uses Werner syndrome model roundworms. Their data show mitophagy in roundworm muscle cells of this model reduce 41% compared to normal roundworms.
The study demonstrates Werner syndrome associates with mitochondrial dysfunction, mainly manifesting as defective mitophagy. Treatment of a Werner syndrome model organism and human cells with NAD+ supplements, NR and NMN, improves mitophagy and increases lifespan. According to Fang et al. (2019), “NAD+ supplementation improves mitochondrial function and other age-related metabolic outcomes.” Mitochondrial health may be a new target in considering interventions for accelerated aging.

